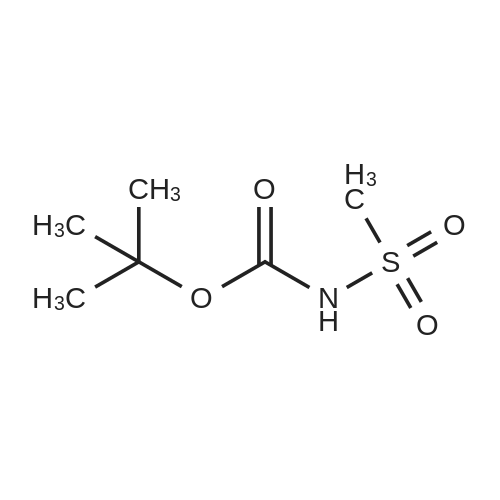
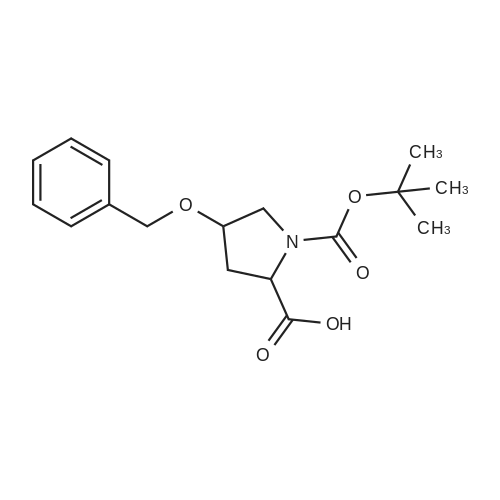
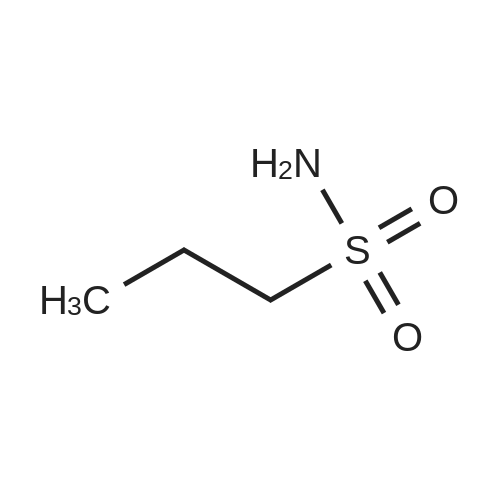
| 26.1 g (85%) |
With hydrogenchloride; dmap; triethylamine; In hexane; dichloromethane; water; ethyl acetate; |
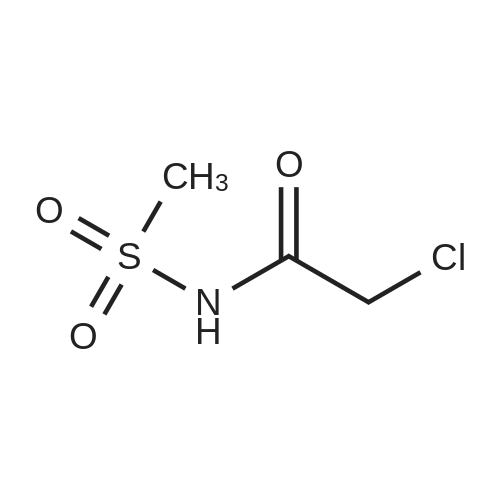
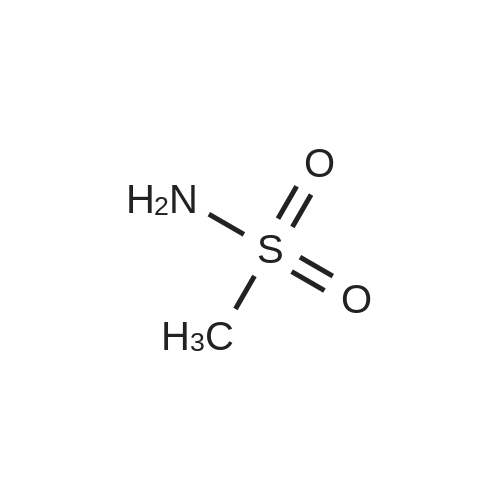
A. N-(t-butoxycarbonyl)methanesulfonamide To a solution of 15.0 g (157.7 mmol) of methanesulfonamide, 17.6 g (173.5 mmol) of triethylamine and 1.9 g (15.8 mmol) of 4-dimethylaminopyridine in 200 mL of dichloromethane was added of 37.9 g (173.5 mmol) of di-t-butyldicarbonate in 200 mL of dichloromethane over ten minutes. The mixture was stirred at ambient temperature for 2.25 hours and concentrated in vacuo. The residue was dissolved in 250 mL of ethyl acetate and washed once with 200 mL of 1 N hydrochloric acid, once with 100 mL of water and once with 100 mL of saturated aqueous sodium chloride. The organic layer was dried (MgSO4), filtered and concentrated in vacuo. The residue was suspended in 100 mL of hexane, filtered and dried in vacuo to afford 26.1 g (85%) of the title compound. Analysis calculated for C7H13NO4S: %C, 36.91; %H, 6.71; %N, 7.17. Found: %C, 36.97; %H, 6.79; %N, 7.04. Mass Spectrum: M+1=196. |
|
|
Methanesulfonamide (4.4g; 46.26 mmol), triethylamine (7.1 ml; 50.88 mmol) and DMAP (565 mg; 4.626 mmol) were dissolved in dry CH2CI2 (50 ml) and stirred at room temperature. A solution of di-tert-butyl dicarbonate (1 l.lg; 53.196 mmol) in dry CH2CI2 (100 ml) was slowly added drop by drop over 10 minutes. After the addition was complete the reaction mixture was stirred a further 1 hour, then the volatiles were removed under reduced pressure. The residues were carefully partitioned with 2N hydrochloric acid (150 ml) and diethyl ether (2 x 150 ml). The ether extracts were combined and washed with brine (150 ml) and the extract dried over anhydrous MgSO4 powder, filtered and the filtrates concentrated under reduced pressure. The solid residues obtained from evaporation was triturated with hexanes, the hexane layer was filtered off and discarded. The remaining solid was crystalized from hexane and diethyl ether to give the title compound. 1H-NMR (400 MHz, CDCl3) delta: 1.53 (s, 9H), 3.28 (s.,3H). |
|
With dmap; triethylamine; In dichloromethane; at 20℃; for 2.5h; |
Reference Example 42 To a mixture of methane sulfonamide (1.96 g), triethylamine (3.2 mL), 4-(dimethylamino)pyridine (252 mg), and dichloromethane (30 mL) was added a mixture of di-tert-butyl dicarbonate (5.17 g) and dichloromethane (40 mL) at room temperature for 30 minutes. The mixture was concentrated after stirring for 2 hours, and the residue was distributed with ethyl acetate and 1 N hydrochloric acid. The organic layer was washed with water, dried and concentrated. The obtained residue was purified by silica gel column chromatography to obtain tert-butyl methylsulfonyl carbamate (2.44 g). 1H-NMR (300 MHz, CDCl3) delta: 1.52 (9H, s), 3.28 (3H, s). |
|
With dmap; triethylamine; In dichloromethane; at 0 - 20℃; |
To a stirred suspension of methylsulfonamide (6 g, 62 mmol) in DCM at 0 C. was added DMAP (760 mg, 6.2 mmol), triethylamine (10.4 ml, 74.4 mmol) and (Boc)2O (14.2 g, 65.1 mmol). The reaction mixture was warmed up to room temperature and stirred overnight. The solution was concentrated and the residue was diluted with ethyl acetate, washed consecutively with 1N HCl and water, dried with Na2SO4, filtered and evaporated to afford a colorless oil. The oil was refluxed in hexane for 1 hour then cooled to room temperature and filtered to afford the target compound as a white solid (12.1 g, 42.1% yield). 1H NMR (DMSO-d6, 300 MHz) delta ppm 11.22 (s, 1H), 3.18 (s, 3H), 1.42 (s, 9H). |
|
With dmap; triethylamine; In dichloromethane; ethyl acetate; |
Triethylamine (22.0 mL, 158 mmol), di-tert-butyl dicarbonate (27.5 g, 126 mmol), and 4-(N,N-dimethylamino)pyridine (1.28 g, 10.5 mmol) were added sequentially to a solution of methanesulfonamide (10.0 g, 105 mmol) in dichloromethane (300 mL) at 25 C. The mixture was stirred at 25 C. for 2 h, and then was concentrated in vacuo to ~40 mL volume. Ethyl acetate (350 mL) was added and the mixture was washed with 1.0 M aqueous hydrochloric acid solution (300 mL). The aqueous layer was extracted with ethyl acetate (250 mL) and the combined organic layers were dried over sodium sulfate, filtered and were concentrated in vacuo to afford Boc-N-methanesulfonamide (17.1 g, 87.6 mmol, 83%) as a white solid. 1H NMR (400 MHz, CDCl3) delta: 1.53 (9H, s), 3.27 (3H, s). |
| 1 g |
With dmap; triethylamine; In dichloromethane; at 20℃; for 2h; |
Triethylamine (2.2 mL, 16 mmol), di-tert-butyldicarbonate (2.65 g, 12.1 mmol) and 4-dimethylaminopyridine (0.096 g, 0.79 mmol) were added sequentially to a solution of methanesulfonamide (0.75 g, 7.9 mmol) in methylene chloride (20 mL) at room temperature. The reaction was stirred at room temperature for 2 h and then concentrated. EtOAc was added, and the resultant mixture was washed with 1N aq. HCl solution, dried over MgSO4 and concentrated to give the desired product (1 g) to be used in the next step directly. |
| 26.1 g (85%) |
With hydrogenchloride; dmap; triethylamine; In hexane; dichloromethane; water; ethyl acetate; |
A. N-(t-Butoxycarbonyl)Methanesulfonamide: To a solution of 15.0 g (157.7 mmol) of methanesulfonamide, 17.6 g (173.5 mmol) of triethylamine and 1.9 g (15.8 mmol) of 4-dimethylaminopyridine in 200 mL of dichloromethane was added of 27.9 g (173.5 mmol) of di-t-butyldicarbonate in 200 mL of dichloromethane over ten minutes. The mixture was stirred at ambient temperature for 2.25 hours and concentrated in vacuo. The residue was dissolved in 250 mL of ethyl acetate and washed once with 200 mL of 1 N hydrochloric acid, once with 100 mL of water and once with 100 mL of saturated aqueous sodium chloride. The organic layer was dried (MgSO4), filtered and concentrated in vacuo. The residue was suspended in 100 mL of hexane, filtered and dried in vacuo to afford 26.1 g (85%) of the title compound. Analysis calculated for C7H13NO4S: %C, 36.91; %H, 6.71; %N, 7.17. Found: %C, 36.97; %H, 6.79; %N, 7.04. Mass Spectrum: M+1=196. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping