JAK and mTOR inhibitors prevent cytokine release while retaining T cell bispecific antibody in vivo efficacy
Leclercq, Gabrielle
;
Haegel, Hélène
;
Toso, Alberto
, et al.
J. ImmunoTher. Cancer,2022,10,e003766.
DOI:
10.1136/jitc-2021-003766
PubMed ID:
35064010
More
Abstract: Background: T cell engaging therapies, like chimeric antigen receptor T cells and T cell bispecific antibodies (TCBs), efficiently redirect T cells towards tumor cells, facilitating the formation of a cytotoxic synapse and resulting in subsequent tumor cell killing, a process that is accompanied by the release of cytokines. Despite their promising efficacy in the clinic, treatment with TCBs is associated with a risk of cytokine release syndrome (CRS). The aim of this study was to identify small molecules able to mitigate cytokine release while retaining T cell-mediated tumor killing.
Methods: By screening a library of 52 Food and Drug Administration approved kinase inhibitors for their impact on T cell proliferation and cytokine release after CD3 stimulation, we identified mTOR, JAK and Src kinases inhibitors as potential candidates to modulate TCB-mediated cytokine release at pharmacologically active doses. Using an in vitro model of target cell killing by human peripheral blood mononuclear cells, we assessed the effects of mTOR, JAK and Src kinase inhibitors combined with 2+1 T cell bispecific antibodies (TCBs) including CEA-TCB and CD19-TCB on T cell activation, proliferation and target cell killing measured by flow cytometry and cytokine release measured by Luminex. The combination of mTOR, JAK and Src kinase inhibitors together with CD19-TCB was evaluated in vivo in non-tumor bearing stem cell humanized NSG mice in terms of B cell depletion and in a lymphoma patient-derived xenograft (PDX) model in humanized NSG mice in terms of antitumor efficacy.
Results: The effect of Src inhibitors differed from those of mTOR and JAK inhibitors with the suppression of CD19-TCB-induced tumor cell lysis in vitro, whereas mTOR and JAK inhibitors primarily affected TCB-mediated cytokine release. Importantly, we confirmed in vivo that Src, JAK and mTOR inhibitors strongly reduced CD19-TCB-induced cytokine release. In humanized NSG mice, continuous treatment with a Src inhibitor prevented CD19-TCB-mediated B cell depletion in contrast to mTOR and JAK inhibitors, which retained CD19-TCB efficacy. Ultimately, transient treatment with Src, mTOR and JAK inhibitors minimally interfered with antitumor efficacy in a lymphoma PDX model.
Conclusions: Taken together, these data support further evaluation of the use of Src, JAK and mTOR inhibitors as prophylactic treatment to prevent occurrence of CRS.
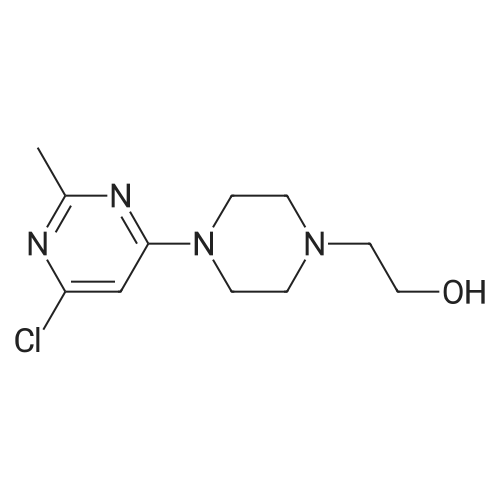
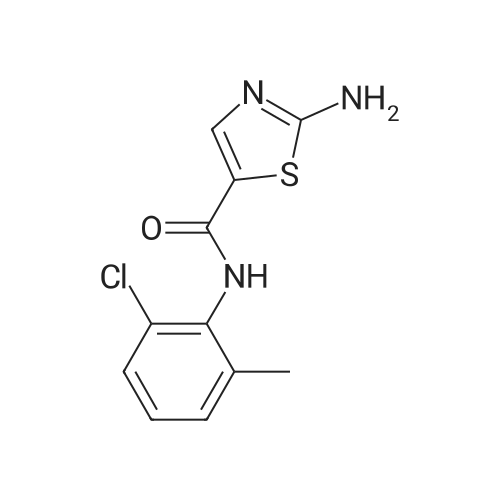
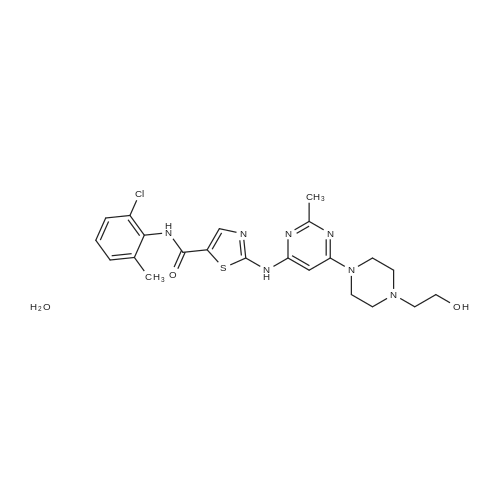
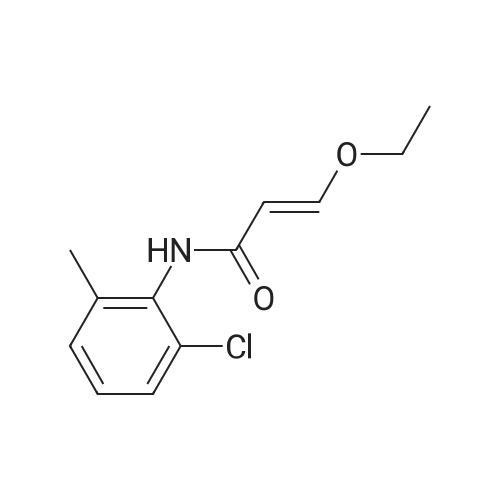
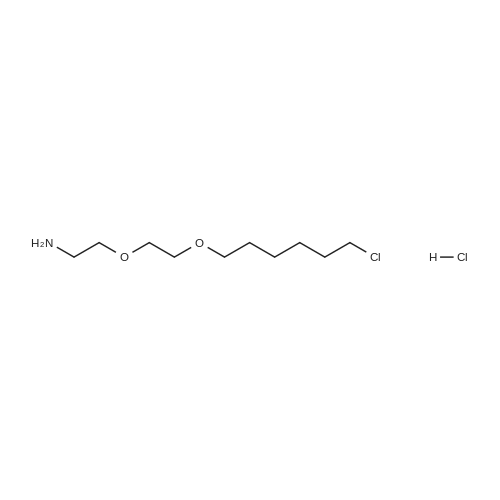
Purchased from AmBeed:
159351-69-6 ;
941678-49-5 ;
302962-49-8 ;
162635-04-3 ;
53123-88-9

Vitamin C Improves Dasatinib Concentrations Under Hypochlorhydric Conditions of the Simulated Stomach Duodenum Model
Fouad S. Moghrabi
;
Aktham Aburub
;
Hala M. Fadda
Pharm. Res.,2022,39(9):2217-2226.
DOI:
10.1007/s11095-022-03321-y
PubMed ID:
35778632
More
Abstract: Purpose:
pH-dependent drug-drug interactions (DDIs) with poorly soluble, weakly basic drugs may lead to clinical implications. Dasatinib is a tyrosine kinase inhibitor with reduced absorption in patients on acid-reducing agents (ARAs). The objective of this study is to investigate the influence of gastric pH on dasatinib supersaturation and determine if vitamin C (L-ascorbic acid) can improve dasatinib concentrations under simulated hypochlorhydric gastric conditions.
Methods:
A dynamic, in vitro, multi-compartment, simulated stomach duodenum (SSD) model mimicking fluid volumes and transfer rates was used to investigate the concentration of BCS class IIb drugs versus time curves. Dasatinib and lamotrigine were explored under normal, fasted, simulated gastric fluids (pH 2) (FaSGF), hypochlorhydric simulated gastric fluids (pH 4.5) (FaSGFhypo) and FaSGFhypo with 1000 mg of vitamin C.
Results:
Significant supersaturation of dasatinib was observed in the duodenum compartment of the SSD model in FaSGF. A 90% reduction in dasatinib AUC∞ was observed in FaSGFhypo. Upon addition of vitamin C to FaSGFhypo, drug concentrations were restored to those observed in FaSGF. Lamotrigine AUC∞ in the duodenal compartment were similar in both FaSGF and FaSGFhypo. The in vitro trends observed for dasatinib and lamotrigine are reflective of the trends observed in vivo in subjects receiving treatment with ARAs.
Conclusions:
The SSD model serves as a good in vitro tool for assessing the effect of pH-dependent DDIs on bioavailability of weakly basic drugs with solubility/ dissolution limited absorption. Vitamin C provides a promising approach for improving bioavailability of poorly soluble, weakly basic drugs in hypochlorhydric patients.
Keywords:
Dissolution ;
Ionization ;
In vitro in?vivo correlations ;
Proton pump inhibitors ;
Oral drug delivery
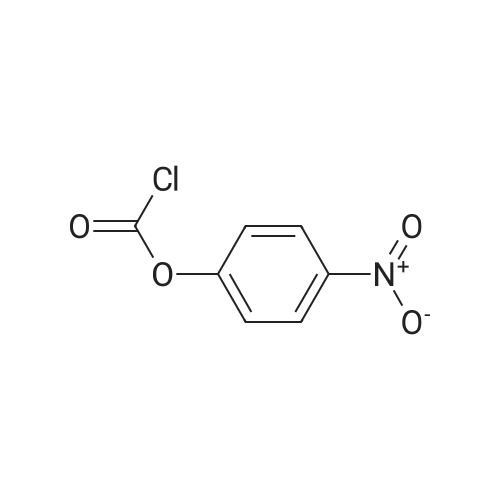
Purchased from AmBeed:
302962-49-8 ;
84057-84-1

Cytokine release in the context of T cell bispecific antibody therapies: biological mechanisms and mitigation strategies
Leclercq, Gabrielle
;
University of Basel,2021.
More
Abstract: T cell bispecific antibodies (TCBs) are a novel class of T cell engagers redirecting T cells towards tumor cells, facilitating the formation of a cytotoxic synapse and resulting in tumor celllysis. On-target activity of TCBs may come with a risk of Cytokine Release Syndrome (CRS), characterized by elevated levels of pro-inflammatory cytokines in the serum, such as IL-6, IL-1β, TNF-α or IFN-γ and over activation of immune cells. Besides, the expression of the tumorassociated antigen on healthy cells may induce off-tumor activity of the TCB and contribute to inflammation. Despite the use of step-up dosing, glucocorticoids, or tocilizumab to manage or prevent these safety liabilities, they remain the major dose-limiting toxicities associated with the treatment of T cell engagers, highlighting the need to develop preventive mitigation treatments. To this aim, we investigated the biological mechanisms and the chronology of events involved in TCB-mediated cytokine release and explored mitigation strategies that might retain profound treatment efficacy while reducing cytokine release. Using an in vitro co-culture of peripheral blood mononuclear cells (PBMCs) or total leukocytes(PBMCs + neutrophils) and target cells with the respective TCBs, or whole blood treated with a B cell depleting TCB, we confirmed the contribution of T cell and myeloid cells and revealed the role of neutrophils in the TCB-mediated cytokine release. In the same model, the use of anticytokine neutralizing antibodies provided insights into the chronology of events triggering the cascade of cytokines after TCB stimulation. Ultimately this work guided the evaluation of mitigation strategies directed against T-cell derived cytokine release by targeting kinases involved in signaling pathways downstream of the T cell receptor (TCR) after stimulation with TCBs. A novel small molecule-kinase inhibitors screen identified mTOR, JAK and Src inhibitors as candidates to switch-off T-cell derived cytokine release. We validated the effects of these kinase inhibitors and fine-tuned their effective doses in in vitro co-cultures of peripheral blood mononuclear cells (PBMCs) and tumor cells with the respective TCBs. In vivo, we used nontumor or tumor-bearing-humanized NSG mice to assess the effect of mTOR, JAK and Srcinhibitors on CD19-TCB-mediated cytokine release and anti-tumor efficacy. Altogether, we confirmed the biological mechanisms of the TCB-mediated cytokine cascade and revealed the contribution of neutrophils. Our work on kinase inhibitors highlights their differential activities on the inhibition of cytokine release and/or T cell cytotoxicity and demonstrates the decoupling between both mechanisms. Our data open new horizons for the prophylactic mitigation of CRS with the use of FDA approved mTOR and JAK inhibitors or the transient use of the Src inhibitor dasatinib. Finally, our results also indicate that dasatinib may serve as an “antidote” against adverse events related to the treatment with TCBs such as high grade CRS or unpredictable off-tumor activity of TCBs, a strategy which is now implemented the clinic.
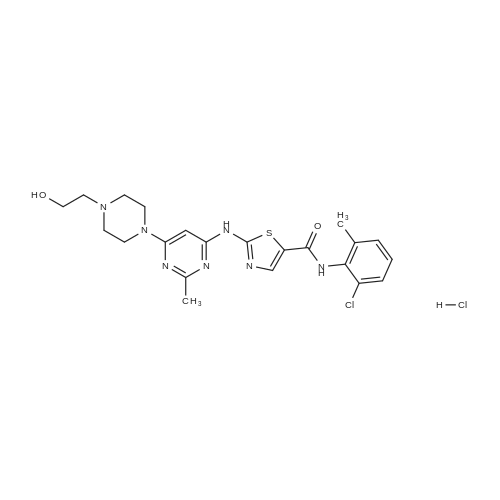
Purchased from AmBeed:
302962-49-8 ;
943319-70-8 ;
162635-04-3 ;
53123-88-9 ;
380843-75-4


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping