| 46.5 mg; 91 mg |
With caesium carbonate; In dichloromethane;Schlenk technique; Inert atmosphere; |
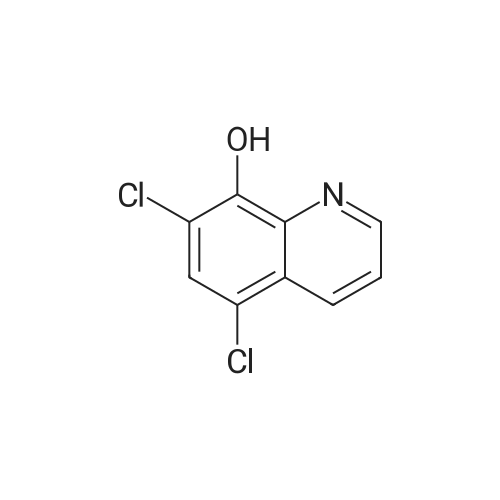
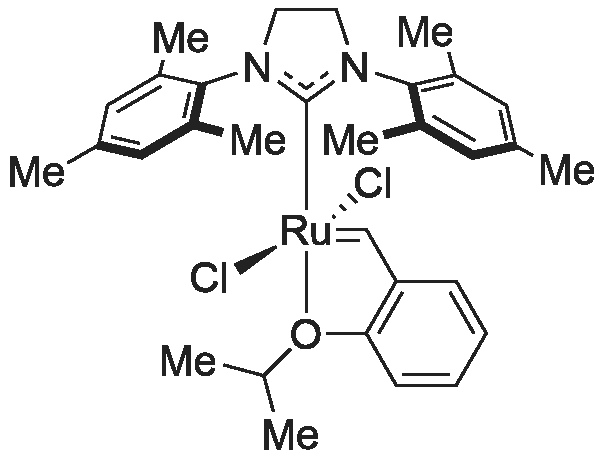
In a Schlenk flask, (H2IMes)Cl2Ru(CH-o-OiPrC6H4) (106 mg, 0.169 mmol, 1 eq) was dissolved in degassed CH2Cl2 (18 mL). <strong>[773-76-2]5,7-Dichloro-8-hydroxyquinoline</strong> (707 mg, 3.303 mmol, 19 eq) and Cs2CO3 (150 mg, 0.461 mmol, 16 eq) were added. The reaction mixture was stirred in a Schlenk flask under argon atmosphere overnight. (0099) The insoluble residue was filtered over celite. According to a TLC (CH/EE 5:1) two derivatives were formed. The products were separated via column chromatography (CH/EE 5:1) and fully characterized by NMR and crystal structure analysis. Yield=83% (46.5 mg 3 and 91 mg 4). (0100) 3: 1H-NMR (delta, 20 C., CDCl3, 300 MHz): 19.10 (s, 1H, Ru?CH), 8.09 (d J=4.04, 1H, CHhq), 7.95 (d J=8.56; j=1.43, 1H, CHhq), 7.68 (d J=8.43 j=1.30, 1H, CHPhq), 7.49 (s, 1H, CHhq), 7.17 (s, 1H, CHhq), 7.05 (m, 2H, CHhq), 6.56 (d J=8.04, 1H, CHhq), 6.48 (s, 2H, CHmes), 6.43 6,39 (?, 2H, CHph), 6.14 (s, 2H, CHmes), 6.06 (2H, CHhq+ph), 3.97 (5H, CH2+CHisoprop), 2.45 (s, 6H), 2.27 (s, 6H), 1.90 (s, 6H, CH31, 1?, 2, 2?, 3, 3?), 1.43 (d, 3H, CH3isoprop), 1.05 (d, 3H, CH3isoprop). (0101) 3: 13C-NMR (delta, 20 C., CDCl3, 75 MHz): 338.6 (1C, Ru?CH), 227.6 (1C, Ru-C), 162.6, 161.3, 149.7, 149.4, 149.0, 144.2, 143.2, 142.4, 142.3, 138.1 (Cq), 136.9 (Cq), 136.6 (Cq), 135.8 (Cq), 132.3 (CH), 131.7 (CH), 129.3 (CH), 129.2 (CH), 128.7, 127.7 (CH), 126.2, 125.8, 125.7, 122.2 (CH), 121.6 (CH), 121.0 (CH), 119.5 (CH), 118.9, 112.0, 109.2, 76.2 (1C, CHisoprop), 51.6 (2C, CH2-N), 23.1 (1C, CH3isoprop), 21.5 (1C, CH3isoprop), 20.8, 18.8, 18.5 (2C, CH3mes 7, 7?, 8, 8?, 9, 9?). (0102) 4: 1H-NMR (delta, 20 C., CDCl3, 300 MHz): 18.23 (bs, 1H, Ru?CH), 9.00 (d j=4.67 Hz, 1H, CHhq 1), 8.09 (d J=8.56 Hz, 1H, CHhq 3), 7.83 (d J=8.30 Hz, 1H, CHhq 3) 7.57 (s, 1H, CHhq 4 or 4), 7.12 (s, 1H, CHhq 4 or 4), 7.06 (q, 1H, CHhq 2), 6.94 (t, 1h; CHph 3 or 4), 6.59 (s, 2H, CHmes 3+3? or 5+5?), 6.39 (d, 1H, CHph 2 or 5), 6.26 (s, 2H, CHmes 3+3? or 5+5?), (d, 1H, CHph 2 or 5), (t, 1H, Chhq 2), 5.98 (t, 1H, CHph 3 or 4), 5.32 (d j=4.54 Hz, 1H, CHhq 1), 4.54 (m, 1H, CHisoprop), 3.92 (q, 4H, CH2mes), 2.57 (s, 6H), 2.04 (s, 6H), 1.91 (s, 6H, CH3mes 7, 7?, 8, 8?, 9, 9?), 1.53 (d, 3H, CH3isoprop), 1.31 (d, 3H, CH3isoprop). (0103) 13C-NMR (delta, 20 C., CDCl3, 75 MHz): Ru?C not observed, 209.5 (1C, Ru-C), 166.4 (Cq), 160.9 (Cq), 147.7 (Cq), 146.7 (Cq), 147.1 (Cq), 146.7 (Cq), 164.5 (CH), 146.5 (CH), 144.9 (Cq), 141.2 (CH), 137.1 (Cq), 137.0 (Cq), 136.7 (Cq), 136.5 (Cq), 119.3 (Cq), 125.8 (Cq), 132.7 (CH), 132.2 (CH), 129.2 (CH), 129.1 (2C, CH), 129.0 (CH), 128.6 (CH), 127.9 (CH), 126.4 (Cq), 120.7 (CH), 120.1 (CH), 119.7 (CH), 118.0 (Cq), 111.3 (Cq), 110.5 (CH), 106.4 (Cq), 68.7 (1C, CHisoprop) 51.7 (2C, CH2), 22.7, 22.3 (2C, CH3isoprop), 20.9, 18.9, 18.1 (6C, CH3mes 7, 7?, 8, 8?, 9?). (0104) Even if both of the catalysts possess two <strong>[773-76-2]5,7-dichloro-8-hydroxyquinoline</strong>s, they show different NMR patterns. The different structures were revealed by X-ray diffraction. The crystals for the X-ray diffraction measurement were obtained by slow diffusion of Et2O in a saturated solution of CH2Cl2. The two derivatives exhibit a different geometry considering the 8-quinolinolate substituents. In derivative 3, the oxygen atoms of the two quinolinolates are orientated trans to each other, while in derivative 4 these trans positions are occupied by an oxygen and a nitrogen atom of the two different quinolinolates. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping