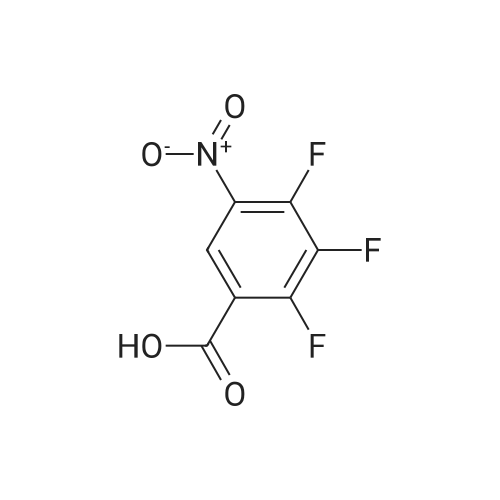
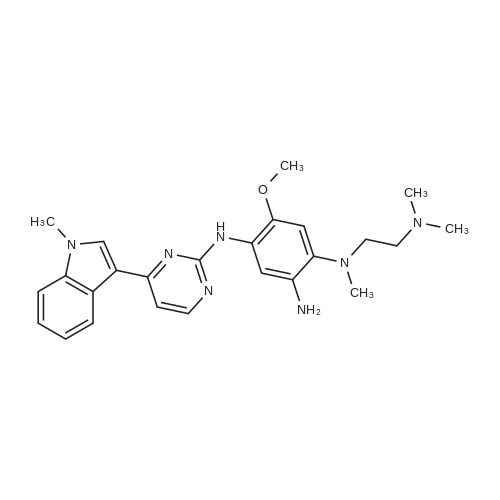
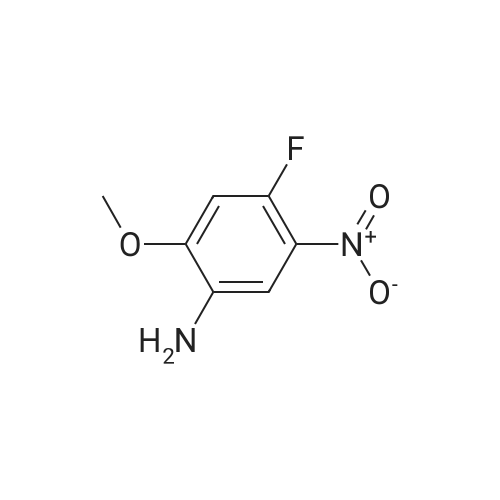
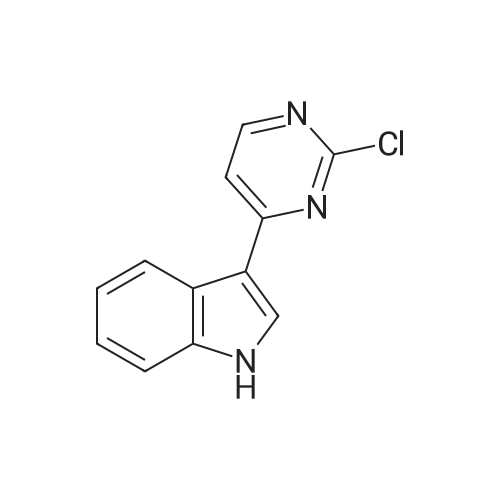
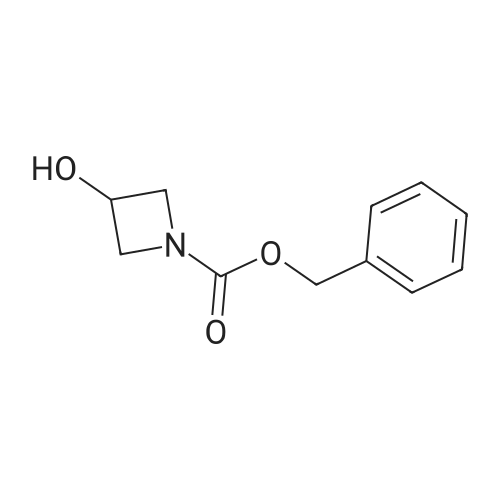
| 94% |
With potassium phosphate; copper(l) iodide; N,N`-dimethylethylenediamine; In toluene; at 160℃; under 4500.45 - 5250.53 Torr; for 2h;Microwave irradiation; |
General procedure: Microwave-assisted Ullmann-Goldberg coupling of 1.0 equivalent of the corresponding lactams(compound 1-4) with 1.2 equiv. of 2-fluor-4-iodoaniline (5), 0.5 equiv. of CuI (except for compound 1where 2 equivalents of catalyst were used), 2 equivalents of N,N’-dimethlyethylenediamine (DMEDA),and 2 equivalents of K3PO4 in 3.5 mL of dry toluene. The reaction mixture was irradiated in a microwaveoven with 6-7 bar, 850 watts for 2 h at 160 C (except for compound 7, where 90 C was used).The resulting solution was passed through a pad of silica and Celite with ethyl acetate and thecrude mixture was purified by flash column chromatography (silica gel, n-hexane/AcOEt, 5:5) [80]. |
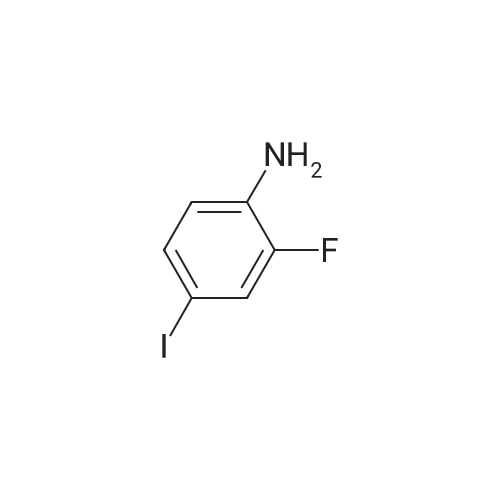
| 74% |
With potassium phosphate; copper(l) iodide; N,N`-dimethylethylenediamine; In 1,4-dioxane;Reflux; Inert atmosphere; |
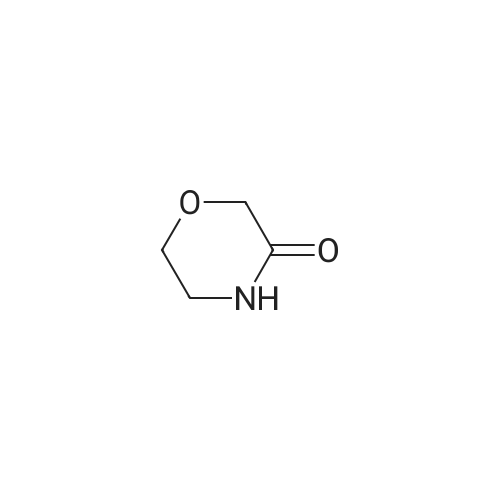
Under argon, a suspension of 29.6 g (125 mmol) of 2-fluoro-4-iodoaniline, 15.8 g (156 mmol, 1.25 eq.) of <strong>[109-11-5]morpholin-3-one</strong> [J.-M. Lehn, F. Montavon, Helv. Chim. Acta 1976, 59, 1566-1583], 9.5 g (50 mmol, 0.4 eq.) of copper(I) iodide, 53.1 g (250 mmol, 2 eq.) of potassium phosphate and 8.0 ml (75 mmol, 0.6 eq.) of N,N'-dimethylethylenediamine in 300 ml of dioxane is stirred under reflux overnight. After cooling to RT, the reaction mixture is filtered through a layer of kieselguhr, and the residue is washed with dioxane. The combined filtrates are concentrated under reduced pressure. The crude product is purified by flash chromatography (silica gel 60, dichloromethane/methanol 100:1→100:3). This gives 24 g (74% of theory) of the title compound. LC-MS (method 4): Rt=0.87 min; MS (ESIpos): m/z=211 [M+H]+; 1H-NMR (500 MHz, DMSO-d6): δ=7.05 (dd, 1H), 6.87 (dd, 1H), 6.74 (dd, 1H), 5.14 (s, 2H), 4.11 (s, 2H), 3.92 (dd, 2H), 3.63 (dd, 2H). |
| 74% |
With potassium phosphate; copper(l) iodide; N,N`-dimethylethylenediamine; In 1,4-dioxane;Reflux; Inert atmosphere; |
Method 2:Under argon, a suspension of 29.6 g (125 mmol) of 2-fluoro-4-iodoaniline, 15.8 g (156 mmol, 1.25 eq.) of <strong>[109-11-5]morpholin-3-one</strong> [J.-M. Lehn, F. Montavon, Helv. Chim. Acta 1976, 59, 1566-1583], 9.5 g (50 mmol, 0.4 eq.) of copper(I) iodide, 53.1 g (250 mmol, 2 eq.) of potassium phosphate and 8.0 ml (75 mmol, 0.6 eq.) of N,N'-dimethylethylenediamine in 300 ml of dioxane was stirred under reflux overnight. After cooling to RT, the reaction mixture was filtered through a layer of kieselguhr and the residue was washed with dioxane. The combined filtrates were concentrated under reduced pressure. The crude product was purified by flash chromatography (silica gel 60, dichloromethane/methanol 100:1→100:3). This gave 24 g (74% of theory) of the title compound.LC-MS (Method 3): Rt=0.87 min;MS (ESIpos): m/z=211 [M+H]+;1H-NMR (500 MHz, DMSO-d6): δ=7.05 (dd, 1H), 6.87 (dd, 1H), 6.74 (dd, 1H), 5.14 (s, 2H), 4.11 (s, 2H), 3.92 (dd, 2H), 3.63 (dd, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping