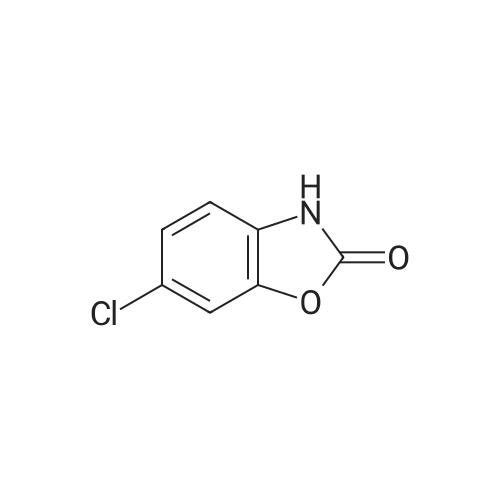
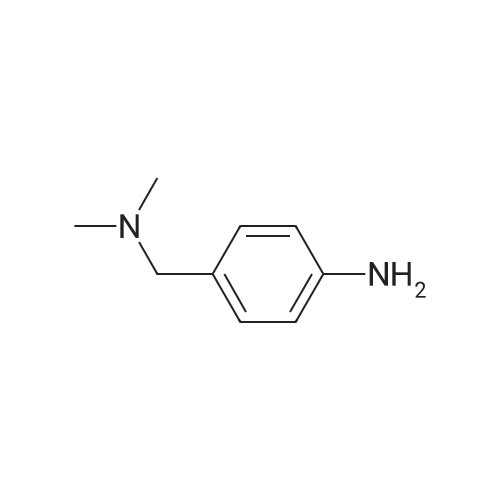
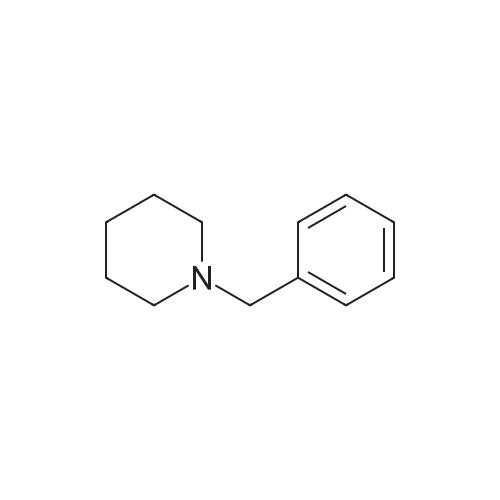
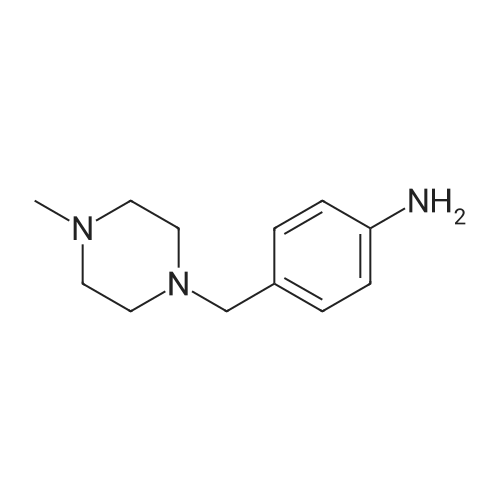
| 78% |
With hydrogenchloride; tin; at 70℃; for 2h; |
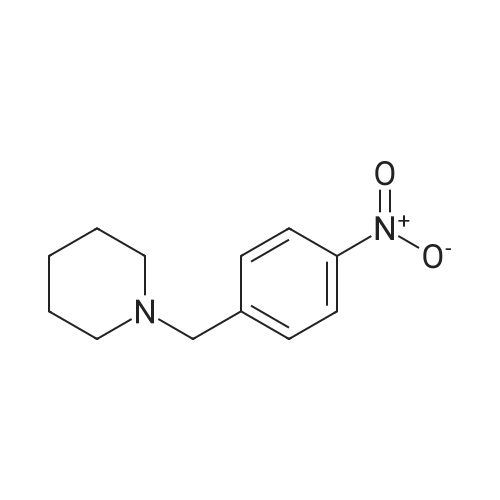
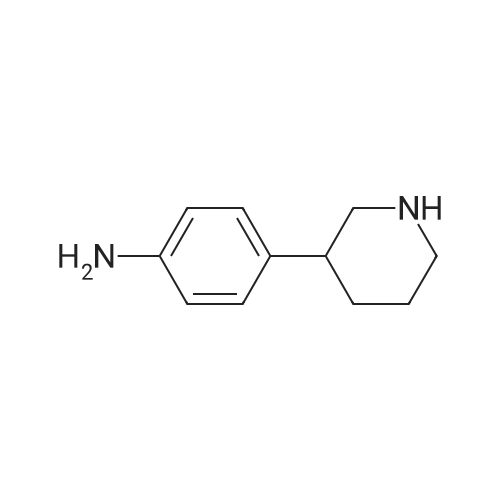
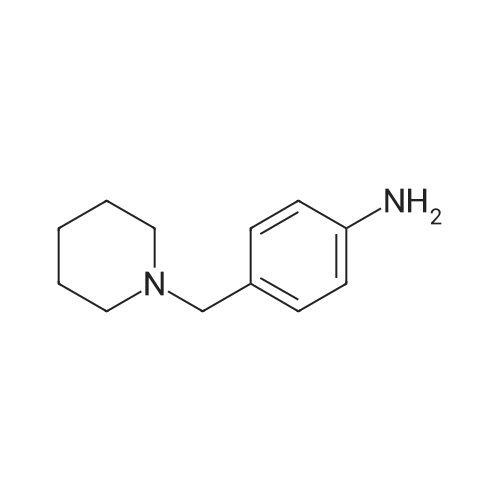
General procedure: To a solution of 1-(chloromethyl)-4-nitrobenzene 1 (2mmol, 1 equiv.) in toluene was added slowly the corresponding dialkylamine or tert-butylamine (10mmol, 5 equiv.). The mixture was stirred under reflux for 4-5h and monitored by TLC using solvent mixture Hex/AcOEt (7:3). The reaction mixture was then cooled to room temperature, the solvent and the unreacted alkyl (dialkyl)amine was evaporated under reduced pressure to give a yellow-white solid of 1-(alkyl (dialkyl)amine-methyl)-4-nitrobenzenes 2a-e (see 1H-NMR and 13C-NMR data in supplementary data from page 8 to page 23), which was pure enough to proceed with the oxidation step. These 1-(dialkylaminemethyl)-4-nitrobenzene 2a-e (2mmol, 1 equiv.) were treated with a solution of hydrochloric acid (20mmol) and tin powder (6mmol, 3 equiv.) under reflux for 2h. The reaction mixture was then cooled to room temperature and neutralized carefully with sodium hydroxide solution (aq. 10%) and extracted with dichloromethane (3×20mL). The organic lawyer was washed with distilled water, dried using anh. MgSO4, filtered and the solvent evaporated under reduced pressure to give the aniline product, which was purified by flash chromatography column using as eluent dichloromethane: methanol (9/1) to give a yellow-orange compound 3a-e. |
| 75% |
With hydrogen;nickel; In methanol; at 20℃; under 2250.23 Torr; for 1.41667h; |
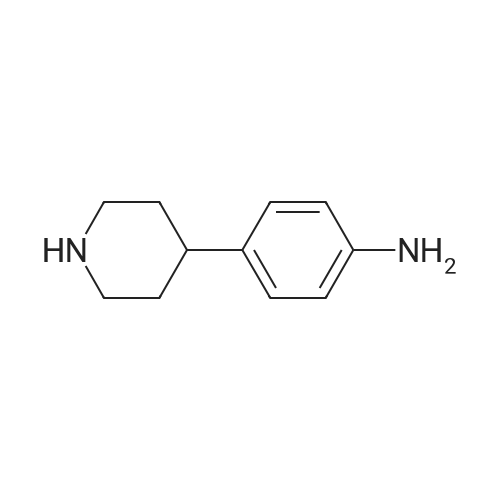
[00922] 37.0 g of 4-(piperidin-1-yl-methyl)-nitrobenzene are dissolved in 300 ml of methanol, 8.0 g of Raney nickel are added and the mixture is hydrogenated for 85 minutes with 3 bars of hydrogen at room temperature. The catalyst is filtered off and the filtrate is evaporated down. [00923] Yield: 24.0 g (75% of theory), Rf value: 0.4 (silica gel, methylene chloride/methanol=9:1) C12H18N2. [00924] ESI mass spectrum: m/z=191 [M+H+]. |
| 75% |
With hydrogen;nickel; In methanol; at 20℃; under 2250.23 Torr; for 1.41667h; |
37.0 g of 4-(piperidin-1-yl-methyl)-nitrobenzene are dissolved in 300 ml of methanol, 8.0 g of raney nickel are added and the mixture is hydrogenated for 1 hour 25 minutes with 3 bar hydrogen at ambient temperature.. The catalyst is filtered off and the filtrate is concentrated by evaporation. Yield: 24.0 g (75% of theory), Rf value: 0.4 (silica gel, methylene chloride/methanol=9:1) C12H18N2 ESI mass spectrum: m/z=191 [M+H+] |
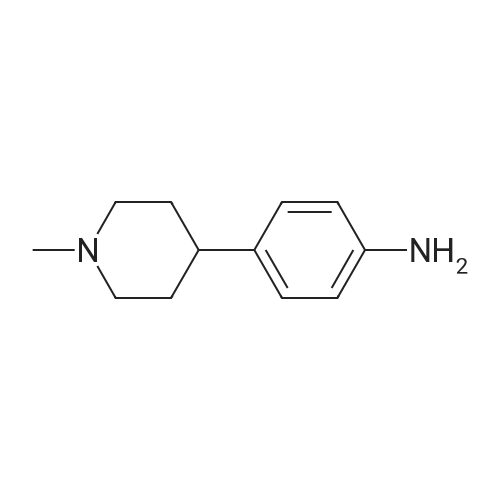
| 59% |
With 5%-palladium/activated carbon; hydrogen; In methanol; at 20℃; |
General procedure: To a solution of 1-(bromomethyl)-4-nitrobenzene 4 (1.0 g, 4.6 mmol) in 15 mL acetonitrile, was added a solution of piperidine in 5 mL acetonitrile at room temperature. The mixture was refluxed for 2 h and the solvent was removed under vacuum to nearly dryness. The residue was acidified with 20mL 2mol/L hydrochloric acid and extracted with ethyl acetate (10mL×3). Concentrated ammonium hydroxide was added to aqueous solution up to clearly basic, and the product was extracted with ethyl acetate (20mL×3). The combined organic layer was washed with brine, dried over anhydrous Na2SO4, and removed solvent under reduced pressure to give 5a which was used in the following reaction without further purification. A mixture of 5a (1.0 mmol) in methanol (20 mL) and 5% palladium on carbon (20 mg) was stirred under hydrogen (balloon) for 6-10 h. The mixture was then filtered through celite and then concentrated to obtain crude product, which was purified by silica gel column chromatography eluting with petroleum, ethyl acetate and triethylamine (20:20:1) to afford 6a. |
|
With hydrogen;palladium 10% on activated carbon; In ethyl acetate; under 2585.81 Torr; for 2h; |
10% Pd/C (300 mg) was added to a solution of Compound Ij (5.0 g, 23 mmol) in EtOAc (50 mL). The mixture was hydrogenated at 50 psi for a period of 2 hrs and filtered through Celite. The filtrate was evaporated and the residue was dissolved in 10% NH4Cl and washed with ethyl ether. The aqueous layer was then adjusted to pH 10 with NaOH and extracted with EtOAc. The combined organic layers were dried over MgSO4, then filtered, EPO <DP n="91"/>evaporated in vacuo and isolated from hexanes to provide 4-piperidin-l-ylmethyl-phenylamine Compound Ik (3.5 g) as an off-white solid. MS 191 (MH+). |
|
With hydrogen;Raney Ni; In tetrahydrofuran; at 20℃; under 760.051 Torr; for 12h; |
Procedure W: Intermediate 28 (1-28) - 4-(Piperidin-l-ylmethyl)benzenamine.; [00145] To a solution of 0.22 g (1.0 mmol, 1.0 eq.) of l-(4-nitrobenzyl)piperidine (I- 27) in 20 mL of THF was added ~30 mg of Raney Nickel. The reaction mixture was stirred under at room temperature under 1 atm. of hydrogen for 12 h. The reaction mixture was through Celite and the solvent removed in vacuo to provide 4- (piperidin-1 -ylmethyl)benzenamine (1-28). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping