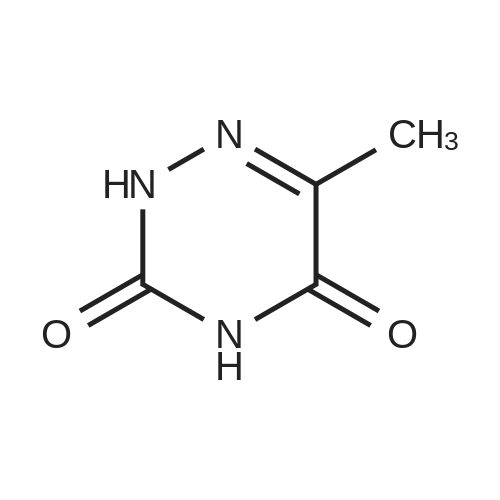
| 49% |
With sodium hydroxide; In methanol; water; at 50 - 55℃; for 17h; |
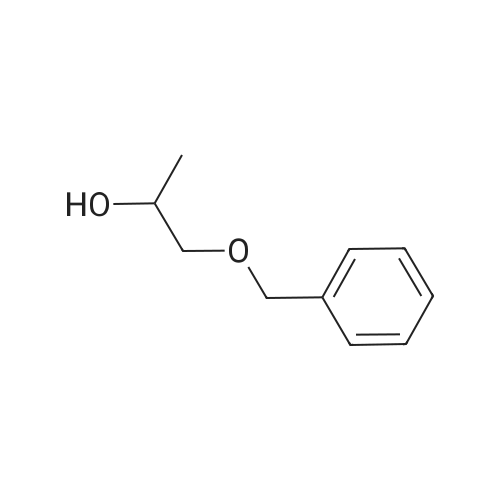
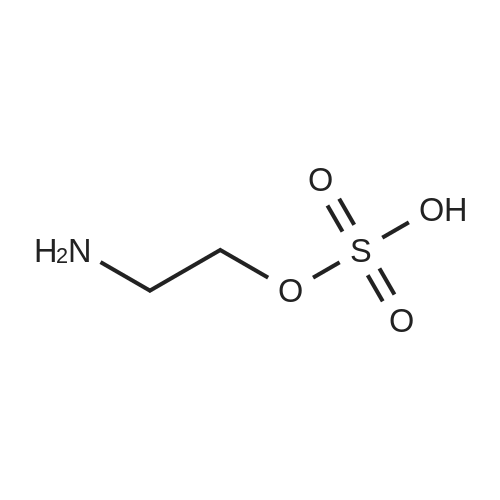
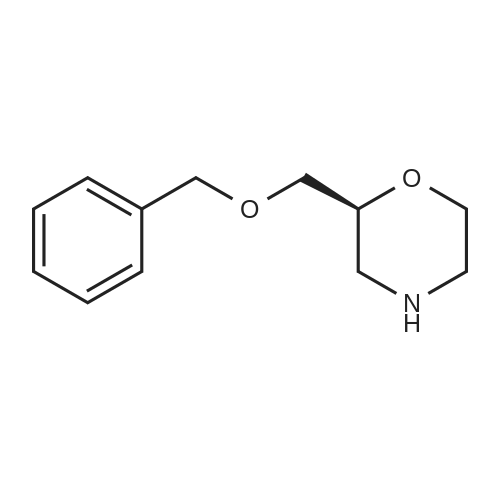
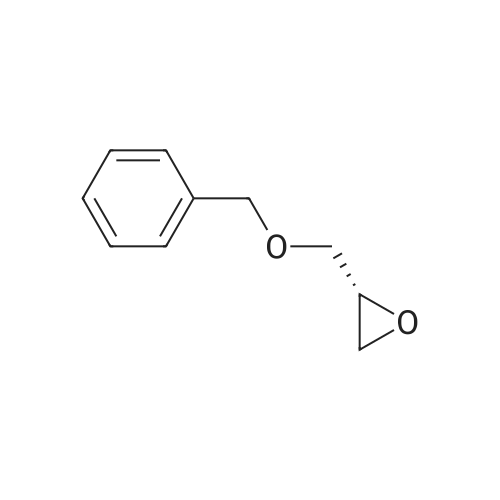
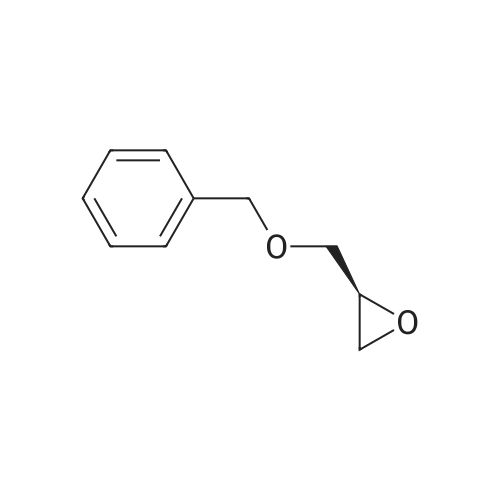
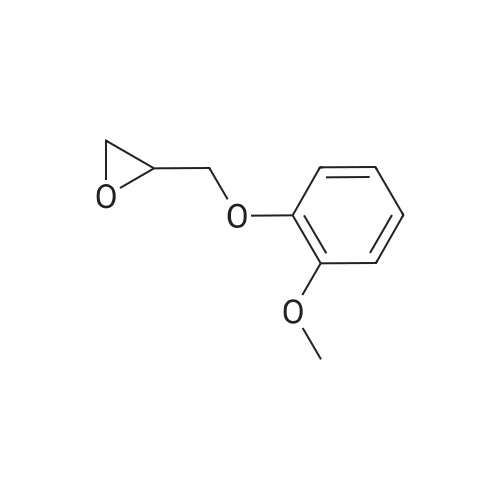
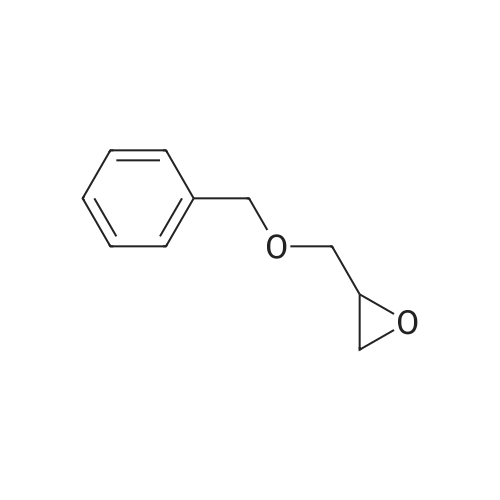
Example 4; (R)-Quinuclidin-3-yl 5-((R)-2-((4-amino-5-chloro-2-methoxybenzamido)methyl)morpholino)hexanoate [Show Image] Mono-(2-amino-ethyl) sulfate (35.2 g, 250 mmol) was dissolved in 60 mL of aqueous sodium hydroxide solution (40percent) with stirring. The solution of (S)-benzyl glycidyl ether 4a (8.2 g, 50 mmol) in methanol was added to the above sodium hydroxide solution. After reacting for 1 hour at 50°C, 100 mL of aqueous sodium hydroxide solution (40percent) was added. The reaction mixture was stirred at 50-55°C for 16 hours and monitored by thin layer chromatography until the disappearance of the starting materials. The resulting mixture was diluted with 100 mL of water and 100 mL of concentrated hydrochloric acid was added dropwise. The mixture was extracted with dichloromethane (500 mL.x.4). The combined organic phase was washed successively with water (300 mL) and saturated brine (300 mL), dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain the title compound (S)-2-benzyloxymethyl-morpholine 4b (5.08 g, yield 49percent) as a yellow liquid. MS m/z (ESI): 208.7[M+1]. 1H NMR (CDCl3, 400 MHz) delta 7.4-7.2(m, 5H), 4.56 (s, 2H), 3.9 (d, 1H, J=11Hz), 3.8-3.35 (m, 4H), 2.92 (dd, 1H, J1=2.5Hz, J2=12.0Hz), 2.85-2.75 (m, 2H), 2.66 (dd, 1H, J1=10.5Hz, J2=12.0Hz), 2.4 (s, 1H). |
| 49% |
With sodium hydroxide; In methanol; water; at 50 - 55℃; for 17h; |
Mono-(2-amino-ethyl) sulfate (35.2 g, 250 mmol) was dissolved in 60 mL of aqueous sodium hydroxide solution (40percent) with stirring. The solution of (S)-benzyl glycidyl ether 4a (8.2 g, 50 mmol) in methanol was added to the above sodium hydroxide solution. After reacting for 1 hour at 50° C., 100 mL of aqueous sodium hydroxide solution (40percent) was added. The reaction mixture was stirred at 50-55° C. for 16 hours and monitored by thin layer chromatography until the disappearance of the starting materials. The resulting mixture was diluted with 100 mL of water and 100 mL of concentrated hydrochloric acid was added dropwise. The mixture was extracted with dichloromethane (500 mL.x.4). The combined organic phase was washed successively with water (300 mL) and saturated brine (300 mL), dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain the title compound (S)-2-benzyloxymethyl-morpholine 4b (5.08 g, yield 49percent) as a yellow liquid.MS m/z (ESI): 208.7 [M+1].1H NMR (CDCl3, 400 MHz) delta 7.4-7.2 (m, 5H), 4.56 (s, 2H), 3.9 (d, 1H, J=11 Hz), 3.8-3.35 (m, 4H), 2.92 (dd, 1H, J1=2.5 Hz, J2=12.0 Hz), 2.85-2.75 (m, 2H), 2.66 (dd, 1H, J1=10.5 Hz, J2=12.0 Hz), 2.4 (s, 1H). |
|
|
In a 500 mL flask were combined (S)-benzyl glycidyl ether (15g, 91.4 rniriol) , MeOH (10 mL) , and 50percent wt. NaOH (30 mL, 365 mmol) . To this mixture was added 2-aminoethylsulfate (25.8 g, 183 mmol) in portions . This heterogeneous mixture was heated to 4O0C at which point the solution becomes homogenous. The temperature was maintained at 400C for 4 h. The reaction was cooled slightly and additional solid NaOH (14.6 g, 365 mmol) was added along with 50 mL toluene. The biphasic solution then was heated to 65°C for 12 h. The reaction was cooled to room temperature, the layers were separated and the aqueous layer was extracted once with EPO <DP n="76"/>75 mli of toluene. The combined organic layers were washed three times with 75 mL portions of IM HCl . The pH of the combined aqueous layers was adjusted to pH 12 with . aqueous NaOH solution and extracted four times with 70 mL portions of EtOAc. The combined organics were dried over Na2SO4 and concentrated in vacuo to yield 10.084 g of the desired morpholine as an opaque oil.The crude morpholine product was dissolved in CH2Cl2 (100 mL) and TEA (12.1 mL, 87.5 mmol) and di-tert- butyl dicarbonate (15.9 g, 73 mmol) was added accompanied by the generation of CO2 gas. The reaction was stirred at room temperature for 18 h, then quenched with 35 mL sat'd aqueous NaHCO3 solution. An additional 50 mL water was added and the layers were separated. The organic layer was dried over anhydrous Na2SO4, concentrated in vacuo and purified by flash chromatography (20percent EtOAc/hexane) to give the desired N-Boc-O-benzyl morpholine as a pale yellow oil (5.536 g) .The purified diprotected morpholine was dissolved in 50 L absolute EtOH and Pd(OH)2 (1.26 g, 20percentwt, 1.8 mmol) was added. A hydrogen balloon was attached and the flask was evacuated using an aspirator and backfilled with H2 three times. The reaction was stirred under H2 for 30 h. The mixture was filtered over celite, rinsing the celite pad thoroughly with EtOH. The filtered solution was concentrated down under vacuum to yield of the desired N-boc-morpholine alcohol as a pale white solid (3.918 g) . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping