| 6.3 g |
In dichloromethane; at 0 - 20℃; for 2h; |
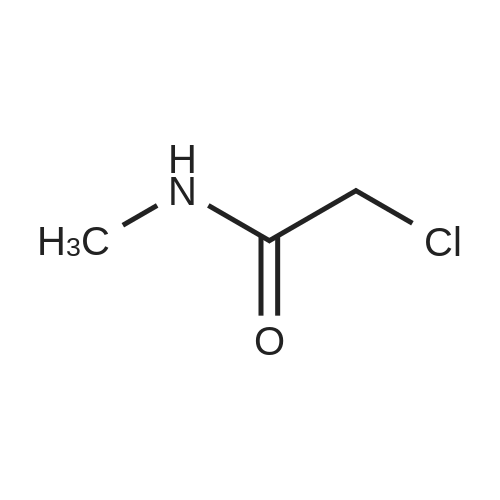
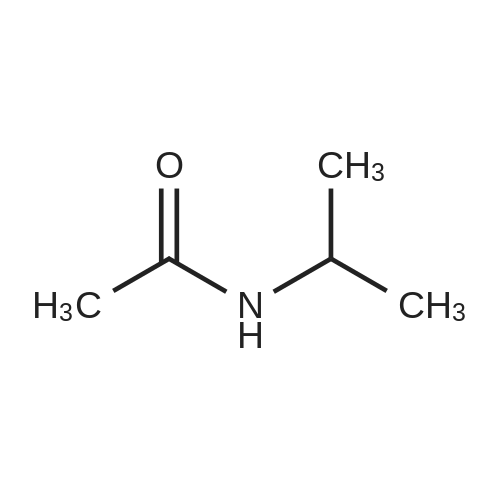
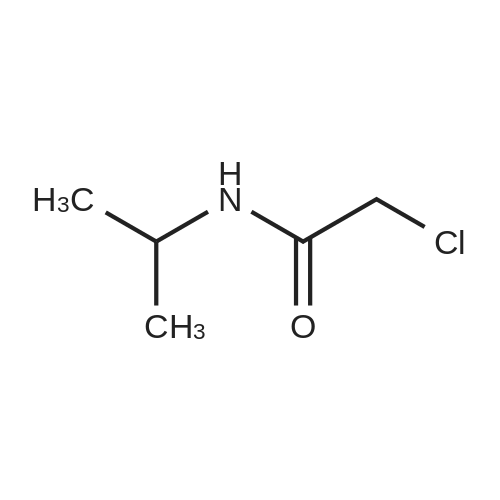
[0094] To a solution of propan-2-amine (5.9 g, 0.1 mol) in DCM (500 rriL) was added 2- chloroacetyl chloride (11.1 g, 0.1 mol) drop wise at 0 C. The mixture was stirred at room temperature for 2 hrs. Then the mixture was quenched with water. The organic phase was washed with saturated brine, dried with anhydrous Na2S04, filtered and concentrated in vacuum to give the product title compound (6.30 g) as a light yellow oil. 1H NMR (400 MHz, DMSO-d6) 5 6.37 (b, 1H), 4.14-4.02 (m, 1H), 1.20 (d, J = 6.8 Hz, 6H). |
|
With potassium carbonate; In dichloromethane; at 20℃;Cooling with ice; |
Isopropylamine (354.7 mg, 6.0 mmol),Potassium carbonate (995.1 mg, 7.2 mmol) was placed in 6 mL of dichloromethane solution,Ice bath,Chloroacetyl chloride (677.6 mg, 6.0 mmol) was slowly added dropwise to the above reaction flask,Room temperature overnight.After the reaction,Add the right amount of water,Extracted three times with methylene chloride,Combined with organic saturated saturated brine,Dried over anhydrous magnesium sulfate,Distillation under reduced pressure to give the crude product as an off-white solid.Yield: 69.9% |
|
With potassium carbonate; In dichloromethane; at 20℃;Cooling with ice; |
General procedure: To a magnetically stirred solution of substituted aniline 1 (50.0 mmol, 1.0 equiv.) and K2CO3 (75.0 mmol, 1.5 equiv.) in CH2Cl2 (100 mL), cooled in an ice bath, the chloroacetyl chloride (60.0 mmol, 1.2 equiv.) was added dropwise slowly. The reaction mixture was stirred at room temperature and monitored by TLC (iodine as streak reagent). After the reaction was completed, the solvent was removed under vacuum and ice water (50 mL) was added into the residue. The mixture was then extracted with ethyl acetate (3 × 50 mL). The organic layers were combined, dried over anhydrous MgSO4, and evaporated under vacuum to give the crude product 2 without further purification. |
|
|
General procedure: Triethylamine (0.3643 g, 3.6 mmol) was added to a solution of the appropriate alkylamine or substituted benzylamine 5a-l (3 mmol) in dichloromethane (7.5 mL), and the reaction mixture was stirred for 5 min at room temperature, then 2-chloroacetyl chloride (0.3857 g, 3.6 mmol) was added dropwise to this reaction mixture at 0 C and stirred for 15 min at room temperature. After completion of the reaction, the solvent was evaporated under reduced pressure to afford 6a-l. KI (0.5976 g, 3.6 mmol) and CTAB (98.40 mg, 7.5% mmol) were added to a solution of the crude product 6a-l in acetone (30 mL) and maintained stirring at reflux for 2 h to afford 7al. K2CO3 (0.2073 g, 3 mmol) was added to a solution of scopoletin (0.3843 g, 2 mmol) in acetone (30 mL), and the reaction mixture was stirred at refluxed for 30 min. Then crude intermediates 7a-l were added into the mixture and maintained reflux for 8-12 h (the reaction progress was monitored by TLC with UV detection). After cooling the reaction and filtration, the solvent was evaporated under reduced pressure, and the residue was dissolved in ethyl acetate, washed with saturation sodium bicarbonate, and saturation salt solution successively, dried over anhydrous sodium sulfate, evaporated under reduced pressure to give the target crude products. The crude products were purified by column chromatography using petroleum ether/ethyl acetate from 6:1 to 2:1 as the gradient eluent system to yield the products 26-37. |
| 440 mg |
With triethylamine; In dichloromethane; at 20℃; for 5h; |
TDI01271-1 (1.0 g, 16.95 mmol) was dissolved in anhydrous dichloromethane (20 mL), and triethylamine (1.88g, 18.64 mmol) and chloroacetyl chloride (2.1 g, 18.64 mmol) were slowly added dropwise. The reaction was performedat room temperature for 5 hours. LC-MS indicated the reaction was complete. The reaction solution was concentratedunder reduced pressure, and the crude product was extracted with saturated dichloromethane (150 mL), and washedwith saturated brine (100 mL). The organic phase was dried over anhydrous sodium sulfate, filtered, and concentratedto afford TDI01271-2 (440 mg, crude product).1H NMR (400 MHz, DMSO-d6) delta 8.13 (s, 1H), 3.99 (s, 2H), 3.87-3.79 (m, 1H), 1.07 (d, J = 6.4 Hz, 6H). MS m/z (ESI):136.2 [M+H]. |
|
With potassium carbonate; In tetrahydrofuran; acetonitrile; at 0 - 20℃; for 3h; |
General procedure: To a solution of 9 (30mmol) in 25mL THF was added K2CO3 (6.2g, 45mmol), and stirred for 20min under the ice bath to make it well mixed. Chloroacetyl chloride (3mL, 36mmol) was added in drops and stirred for 3h under room temperature. The reaction solution was concentrated, water (100mL) was added, and extracted with EtOAc. The organic layer was collected and washed with saturated salt solution for three times, dried over anhydrous Na2SO4 and concentrated in vacuo. The crude material was purified by column chromatography to afford intermediate product 10. |
|
With triethylamine; In dichloromethane;Cooling with ice; |
third step:Isopropylamine,Dichloromethane as a solvent,Add triethylamine (1 mmol),Chloroacetyl chloride (1 mmol) diluted slowly with dichloromethane in a constant pressure dropping funnel under ice conditions, after the reaction is completed, the system is poured into water, the pH is adjusted to weakly basic with Na2CO3, and dichloromethane extraction , The organic phase was concentrated under reduced pressure,The mixture was stirred, filtered, and dried with n-hexane to obtain 2-chloro-N-isopropylacetamide. |
| 6.3 g |
In dichloromethane; at 0 - 20℃; for 2h; |
To a solution of propan-2-amine (5.9 g, 0.1 mol) in DCM (500 mL) was added 2-chloroacetyl chloride (11.1 g, 0.1 mol) drop wise at 0 C. The mixture was stirred at room temperature for 2 hrs. Then the mixture was quenched with water. The organic phase was washed with saturated brine, dried with anhydrous Na2SO4, filtered and concentrated in vacuo to give the product title compound (6.30 g) as a light yellow oil. 1H NMR (400 MHz, DMSO-d6) d 6.37 (b, 1H), 4.14-4.02 (m, 1H), 1.20 (d, J = 6.8 Hz, 6H). |
| 299 mg |
With triethylamine; In dichloromethane; at 0 - 25℃; for 2h;Inert atmosphere; |
To a solution of isopropylamine (200 mg) and dry Et3N (821 mg) in 6.8 mL of dry DCM were added 2-chloroacetyl chloride (458 mg) dropwise at 0 C. After stirring 15 min at this temperature, the mixture was additionally stirred for 2 h at RT. After completion of the reaction as monitored by LCMS water was added and the organic phase was separated. The water phase was extracted two times with DCM and the combined organic layers were dried with MgS04, the solvents were removed under reduced pressure and the crude product was purified by silica gel (0887) chromatography using a gradient of ethyl acetate/cyclohexane as eluent. (0888) Yield: 299 mg MS (ES+) [M+H]+: m/e = 136.0, RT: 0.428 min |
|
With triethylamine; In dichloromethane; at 20℃; |
General procedure: Dissolve different amine compounds in dichloromethane, add triethylamine (2.0 eq.), dropwise add chloroacetyl chloride (1.1 eq.) under an ice bath, and then continue stirring at room temperature for 2-6 h. After completed of the reaction, the mixture was extracted, and recrystallized to obtain intermediates A. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping