| 81% |
With sodium dithionite; In water; at 0 - 30℃; for 1.0h; |
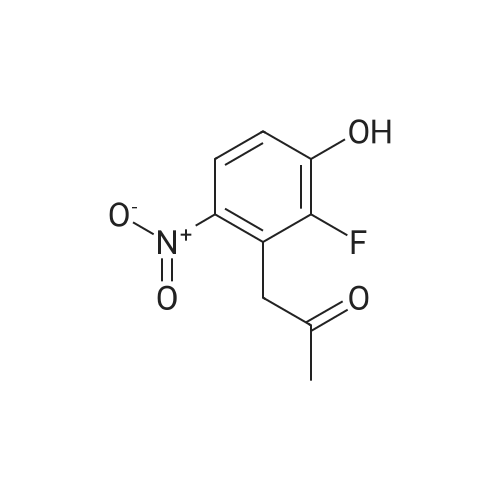
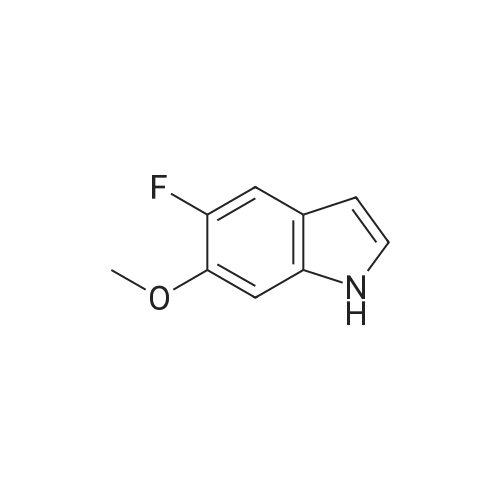
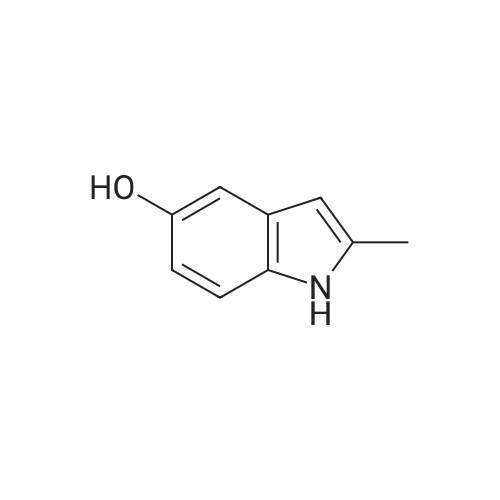
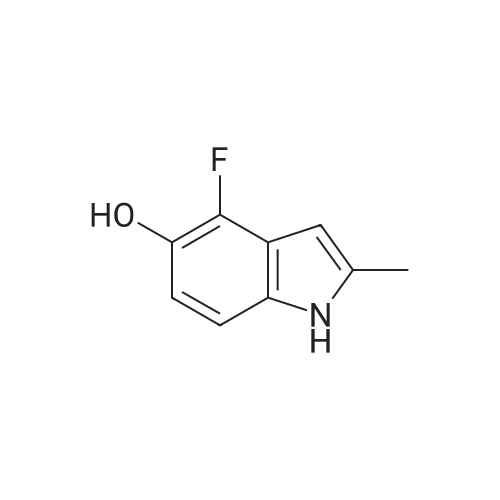
1-(2-fluoro-3-hydroxy-6-nitrophenyl)-propan-2-one from previous step (50 g, 0.234 mol) was added to 2 liter round bottom flask. Water (1L) was added, and the yellow suspension was stirred at Rt. Sodium dithionite (225 g, 5.5 eq) was added in one portion and the reaction mixture was stirred and kept < 30 C until HPLC analysis indicated no starting material remained (typically less than 1 hour). Upon completion, the reaction mixture was cooled to 0 C and the tan solid product was collected by vacuum filtration. The wet product was dried at <50 C under house vacuum to afford 4-fluoro-2-methyl-1H-indol-5-ol (31.4 g, 81 % yield) which was isolated as a tan crystalline powder. The material had an HPLC purity of >99.8. 1H NMR (CDC3, 400 MHz) delta 7.8 (s, 1H), 6.9-6.7 (m, 2H), 6.2 (s, 1H), 4.7 (s, 1H), 2.4 (s, 3H). 13 C NMR (CDCl3, 100 MHz) delta 145.7, 143.4, 137.5, 136.7, 134.4, 120.1, 112.7, 106.8, 95.4, 13.3 |
| 68% |
With sodium dithionite; potassium carbonate; In water; at 25℃; for 2.0h;Industry scale;Product distribution / selectivity; |
Preparation of 4-fluoro-2 -methyl- lH-indol-5-ol (large scale)To a solution of potassium carbonate (79 kg) in water (800 kg) was added l-(2-fluoro-3- hydroxy-6-nitrophenyl)-propan-2-one (61 kg) and the mixture stirred to give a solution. To this solution at 250C was added a solution of sodium dithionite (298 kg) in water (750 kg).The mixture was held at 250C for 2 hours. The product was isolated by filtration, washing the filter cake with water (366 kg). The product was dried under reduced pressure (50 mbar) at350C. Yield: 34 kg, 72%. The crude 4-fluoro-2-methyl-lH-indol-5-ol (33 kg) was dissolved in dichloromethane(880 1) and filtered through silica (33 kg). The filter was washed with dichloromethane (4401). The combined filtrates were distilled, removing 835 1 of distillate. This concentrate was EPO <DP n="43"/>added rapidly to ?hexane (360 kg), resulting in a suspension. The batch was distilled, removing 436 1 of distillate. The batch was cooled to 00C, aged for 1 hour and then filtered. The filter cake was washed with /s°hexane (73 kg). The product was dried under reduced pressure (50 mbar) at 35C. Yield: 31 kg, 68% based on l-(2-fluoro-3-hydroxy-6- s nitrophenyl)-propan-2-one. |
| 17% |
With hydrogen;palladium on activated charcoal; In ethanol; at 20℃; for 8.0h; |
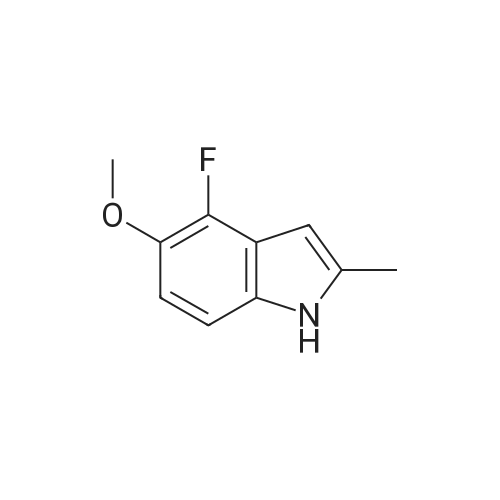
Step 3c: 4-Fluoro-2-methyl-1H-indol-5-ol (Compound 304) A mixture of 303 (900 mg, 4.2 mmol), Pd/C (90 mg) and ethanol (20 mL) was stirred under H2 at ambient temperature for 8 h. The solvent was removed and the residue was purified by column chromatography on silica gel (EtOAc/petroleum ether=1/15) to give the title compound 304 as a brown solid (120 mg, 17%): LCMS: 166 [M+1]+; 1H NMR (DMSO-d6): delta 2.34 (s, 3H), 6.05 (s, 1H), 6.64 (t, J=8.4 Hz, 1H), 6.86 (d, J=8.4 Hz, 1H), 8.70 (s, 1H), 10.84 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping