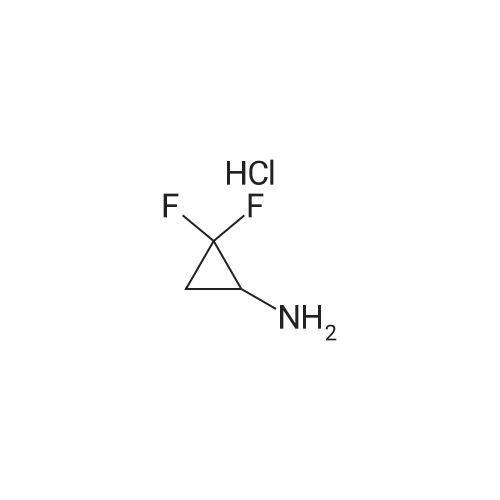
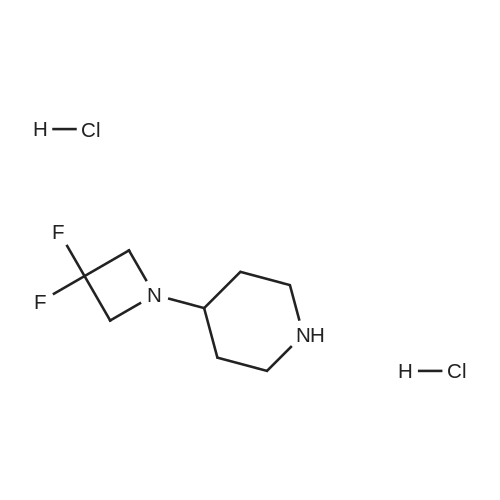
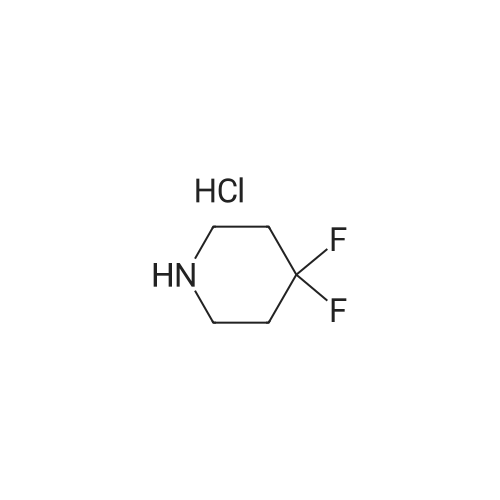
| 88% |
Stage #1: at 20℃; for 0.25 h;
Stage #2: With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane for 17 h; |
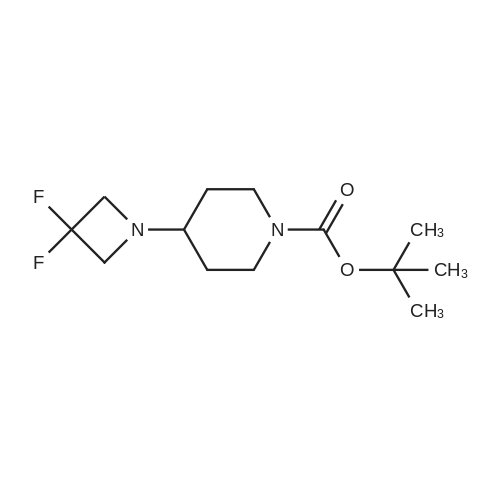
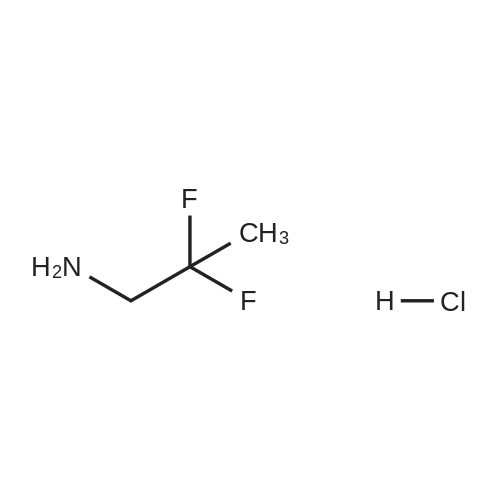
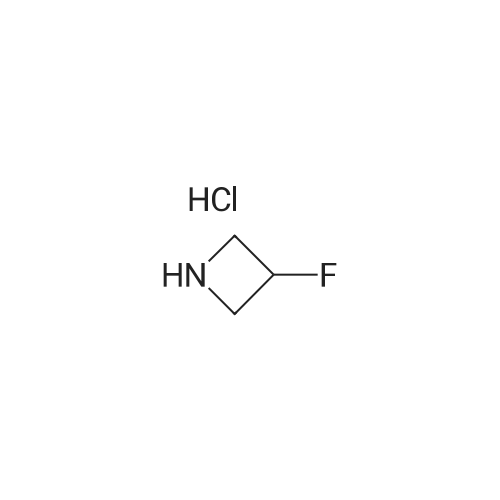
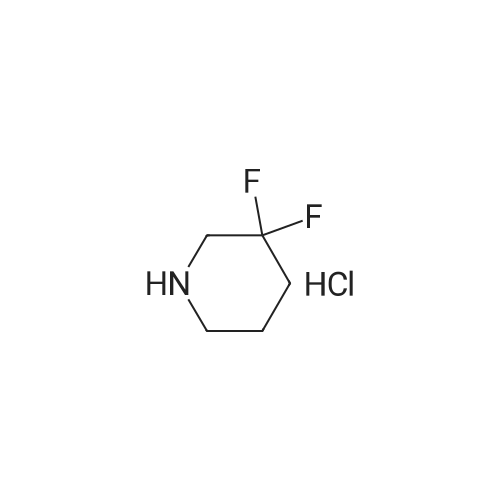
To a solution of 4-oxo-piperidine-l-carboxylic acid tert-butyl ester (1.0 g, 5.0 mmol) in DCE (50 mL) was added 3,3-difluoroazetidine hydrochloride (712 mg, 5.5 mmol). The mixture was stirred at RT for 15 min, then sodium triacetoxyborohydride (1.59 g, 7.5 mmol) was added and stirring was continued for 17 h. The reaction mixture was diluted with brine and extracted with DCM. The organic layer was separated, dried (Na2SO4) and concentrated in vacuo. The resultant residue was purified by column chromatography to give 4-(3,3- difluoroazetidin-l-yl)-piperidine-l-carboxylic acid tert-butyl ester as a pale yellow solid (1.2 g, 88 percent). To a solution of 4-(3,3-difluoro-azetidin-l-yl)-piperidine-l-carboxylic acid tert- butyl ester (552 mg, 2.0 mmol) in DCM (4 mL) was added TFA (2 mL) and the resulting mixture was stirred at RT for 45 min. The reaction mixture was loaded onto an Isolute.(R). SCX- 2 cartridge. The cartridge was washed with MeOH then eluted with 2 M NH3 in MeOH to give the title compound as a pale yellow solid (271 mg, 77 percent). <n="94"/>1H NMR (400 MHz, CDCl3): δ 1.16-1.27 (m, 2 H), 1.63-1.72 (m, 2 H), 2.14-2.23 (m, 1 H), 2.54-2.62 (m, 2 H), 3.09 (dt, J = 12.7, 3.9 Hz, 2 H) and 3.46-3.57 (m, 4 H). |
| 88% |
Stage #1: at 20℃; for 0.25 h;
Stage #2: With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane for 17 h; |
Reference Example 254-(3,3-Difluoro-azetidin-l-yl)-piperidineTo a solution of 4-oxo-piperidine-l-carboxylic acid tert-butyl ester (1.0 g, 5.0 mmol) in DCE (50 mL) was added 3,3-difluoroazetidine hydrochloride (712 mg, 5.5 mmol). The mixture was stirred at RT for 15 min, then sodium triacetoxyborohydride (1.59 g, 7.5 mmol) was added and stirring was continued for 17 h. The reaction mixture was diluted with brine and extracted with DCM. The organic layer was separated, dried (Na2SO4) and concentrated in vacuo. The resultant residue was purified by column chromatography to give 4-(3,3- difluoro-azetidin-l-yl)-piperidine-l-carboxylic acid tert-butyl ester as a pale yellow solid (1.2 g, 88 percent). To a solution of 4-(3,3-difluoro-azetidin-l-yl)-piperidine-l-carboxylic acid tert- butyl ester (552 mg, 2.0 mmol) in DCM (4 mL) was added TFA (2 mL) and the resulting <n="69"/>mixture was stirred at RT for 45 min. The reaction mixture was loaded onto an Isolute.(R). SCX- 2 cartridge, washed with MeOH then eluted with 2 M NH3 in MeOH to give the title compound as a pale yellow solid (271 mg, 77 percent).1H NMR (400 MHz, CDCl3): δ 1.16-1.27 (m, 2 H), 1.63-1.72 (m, 2 H), 2.14-2.23 (m, 1 H), 2.54-2.62 (m, 2 H), 3.09 (dt, J = 12.7, 3.9 Hz, 2 H) and 3.46-3.57 (m, 4 H). |
| 88% |
at 20℃; for 17 h; |
To a solution of 4-oxo-piperidine-l-carboxylic acid tert-butyl ester (1.0 g, 5.0 mmol) in DCE (50 mL) was added 3,3-difluoroazetidine hydrochloride (712 mg, 5.5 mmol). The mixture was stirred at RT for 15 min, then sodium triacetoxyborohydride (1.59 g, 7.5 mmol) was added and stirring was continued for 17 h. The reaction mixture was diluted with brine and extracted with DCM. The organic layer was separated, dried (Na2SO4) and concentrated in vacuo. The resultant residue was purified by column chromatography to give 4-(3,3- difluoroazetidin-l-yl)piperidine-l-carboxylic acid tert-butyl ester as a pale yellow solid (1.2 g, 88 percent). To a solution of 4-(3,3-difluoroazetidin-l-yl)piperidine-l-carboxylic acid tert-butyl ester (552 mg, 2.0 mmol) in DCM (4 mL) was added TFA (2 mL) and the resulting mixture was stirred at RT for 45 min. The reaction mixture was loaded onto an Isolute.(R). SCX-2 cartridge, washed with MeOH then eluted with 2 M NH3 in MeOH to give the title compound as a pale yellow solid (271 mg, 77 percent). 61H NMR (400 MHz, CDCl3): 5 1.16-1.27 (m, 2 H), 1.63-1.72 (m, 2 H), 2.14-2.23 (m, 1 H), 2.54-2.62 (m, 2 H), 3.09 (dt, J = 12.7, 3.9 Hz, 2 H) and 3.46-3.57 (m, 4 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping