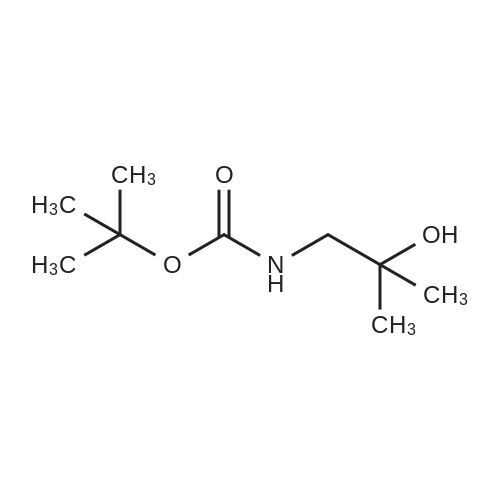
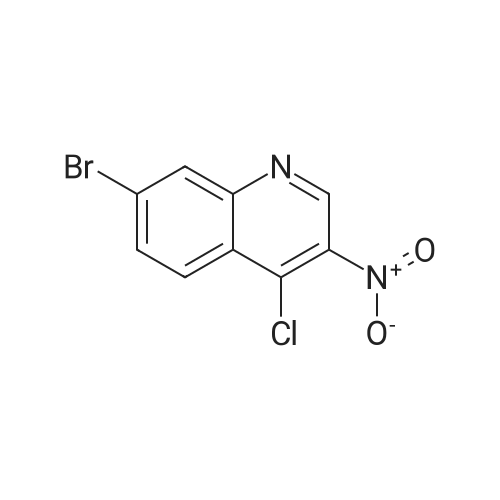
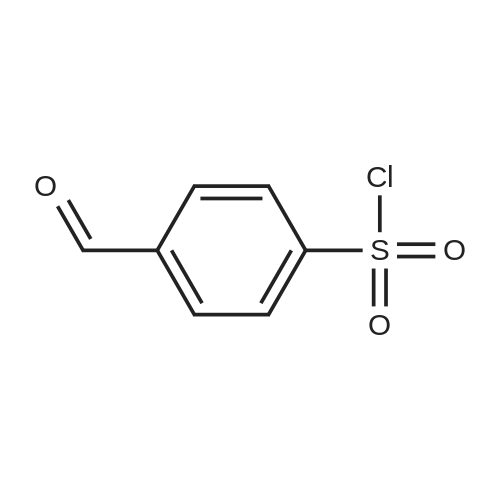
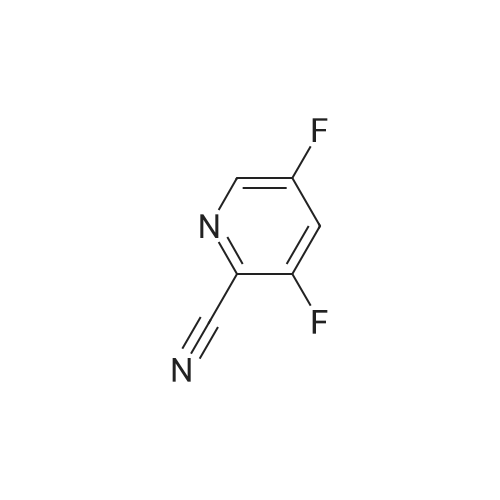
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 75 - 80℃; |
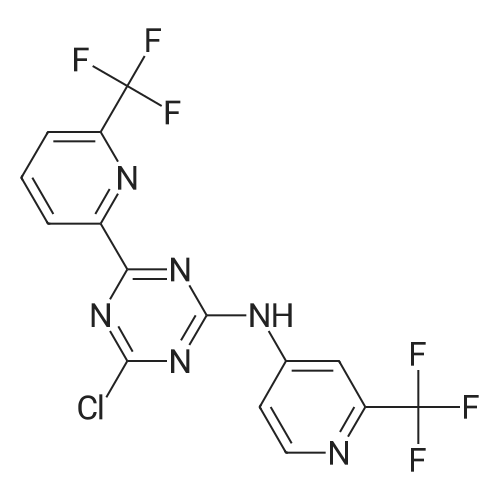
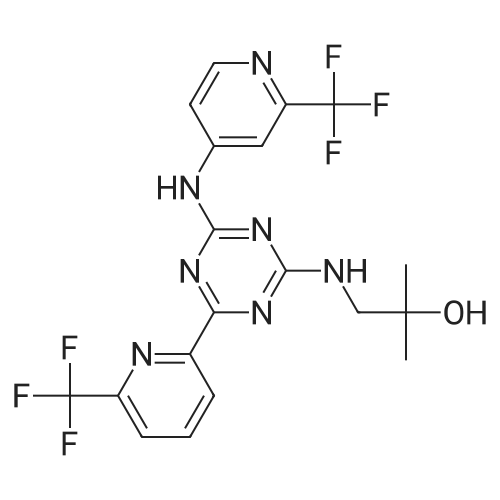
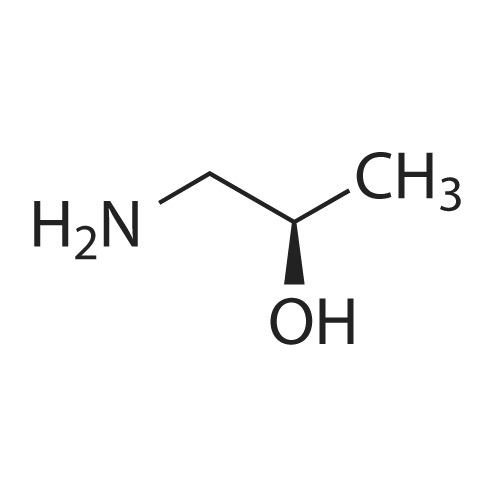
[00274j THF (290 mL), 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)- pyridin-4-yl)-i,3,5-triazin-2-amine (29.0 g, 0.06893 mol), sodium bicarbonate (8.68 g, 0.1033 mol), and 1,1-dimethylaminoethanol (7.37 g, 0.0827 1 mol) are added to the reaction vessel at 20-35 C. The resulting slurry is heated to reflux (75-80 C) for 16-20 h. The reaction is cooled to 30-40 C and THF evaporated at below 45 C under reduced pressure. The reaction mixture is cooled to 20-35 C and rinsed with ethyl acetate and water, and the ethyl acetate layer collected. The organic layer is concentrated under vacuum at below 45 C then rinsed with dichloromethane and hexanes, filtered and washed with hexanes and dried for 8-iOh at 45-50 C under vacuum to provide 2-methyl-i -(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)- pyridin-4-ylamino)- 1,3,5 -triazin-2-ylamino)propan-2-ol. |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 75 - 80℃; |
THF (290 mL), 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)- pyridin-4-yl)-l,3,5-triazin-2-amine (29.0 g, 0.06893 mol), sodium bicarbonate (8.68 g, 0.1033 mol), and 1, 1-dimethylaminoethanol (7.37 g, 0.08271 mol) are added to the reaction vessel at 20-35 C. The resulting slurry is heated to reflux (75-80 C) for 16-20 h. The reaction is cooled to 30-40 C and THF evaporated at below 45 C under reduced pressure. The reaction mixture is cooled to 20-35 C and rinsed with ethyl acetate and water, and the ethyl acetate layer collected. The organic layer is concentrated under vacuum at below 45 C then rinsed with dichlorom ethane and hexanes, filtered and washed with hexanes and dried for 8-1 Oh at 45-50 C under vacuum to provide 2-methyl-l-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2- (trifluoromethyl)- pyridin-4-ylamino)-l,3,5-triazin-2-ylamino)propan-2-ol. |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 20 - 80℃; |
THF (290 mL), 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)-pyridin-4-yl)-l,3 , 5-triazin-2- amine (29.0 g, 0.06893 mol), sodium bicarbonate (8.68 g, 0.1033 mol), and 1,1-dimethylaminoethanol (7.37 g, 0.08271 mol) were added to the reaction vessel at 20-35C. The resulting slurry was heated to reflux (75-80C) for 16-20 h. The reaction was cooled to 30-40C and THF was evaporated at below 45C under reduced pressure. The reaction mixture was cooled to 20-35C, rinsed with ethyl acetate and water, and the ethyl acetate layer was collected. The organic layer was concentrated under vacuum at below 45C then rinsed with dichloromethane and hexanes, filtered and washed with hexanes and dried for 8-10 h at 45-50C under vacuum to provide 2-methyl-i -(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2- (trifluoromethyl)-pyridin-4-ylamino)- 1,3,5 -triazin-2-ylamino)propan-2-ol. |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 20 - 80℃; |
THF (290 mL), 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)-pyridin-4-yl)-1,3,5-triazin-2-amine (29.0 g, 0.06893 mol), sodium bicarbonate (8.68 g, 0.1033 mol), and 1,1-dimethylaminoethanol (7.37 g, 0.08271 mol) were added to the reaction vessel at 20-35 C. The resulting slurry was heated to reflux (75-80 C.) for 16-20 h. The reaction was cooled to 30-40 C. and THF was evaporated at below 45 C. under reduced pressure. The reaction mixture was cooled to 20-35 C., rinsed with ethyl acetate and water, and the ethyl acetate layer was collected. The organic layer was concentrated under vacuum at below 45 C. then rinsed with dichloromethane and hexanes, filtered and washed with hexanes and dried for 8-10 h at 45-50 C. under vacuum to provide 2-methyl-1-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)-pyridin-4-ylamino)-1,3,5-triazin-2-ylamino)propan-2-ol. |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 20 - 80℃; |
Example 2, Step 6: Preparation of 2-methyl-1-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)-pyridin-4-ylamino)-1,3,5-triazin-2-ylamino)propan-2-ol THF (290 mL), 4-chloro-6-(6-(trifluoromethyl)pyridin-2-yl)-N-(2-(trifluoro-methyl)-pyridin-4-yl)-1,3,5-triazin-2-amine (29.0 g, 0.06893 mol), sodium bicarbonate (8.68 g, 0.1033 mol), and 1,1-dimethylaminoethanol (7.37 g, 0.08271 mol) were added to the reaction vessel at 20-35 C. The resulting slurry was heated to reflux (75-80 C.) for 16-20 h. The reaction was cooled to 30-40 C. and THF was evaporated at below 45 C. under reduced pressure. The reaction mixture was cooled to 20-35 C., rinsed with ethyl acetate and water, and the ethyl acetate layer was collected. The organic layer was concentrated under vacuum at below 45 C. then rinsed with dichloromethane and hexanes, filtered and washed with hexanes and dried for 8-10 h at 45-50 C. under vacuum to provide 2-methyl-1-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)-pyridin-4-ylamino)-1,3,5-triazin-2-ylamino)propan-2-ol. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping