|
|
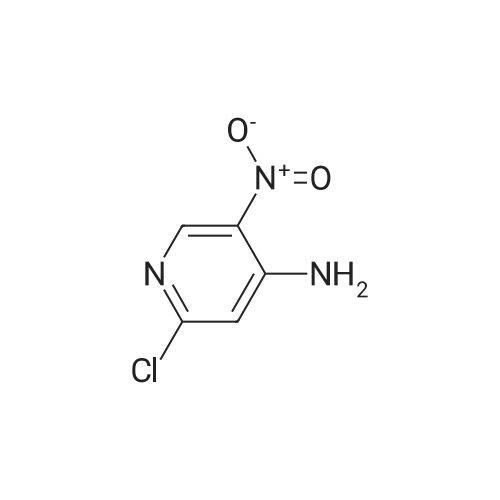
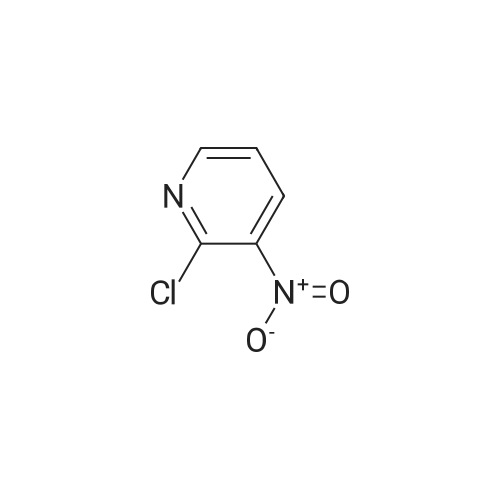
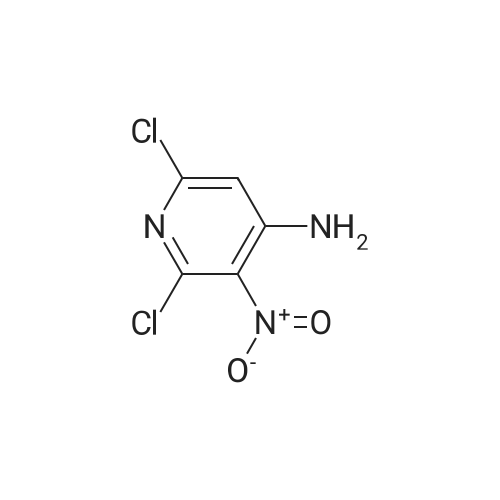
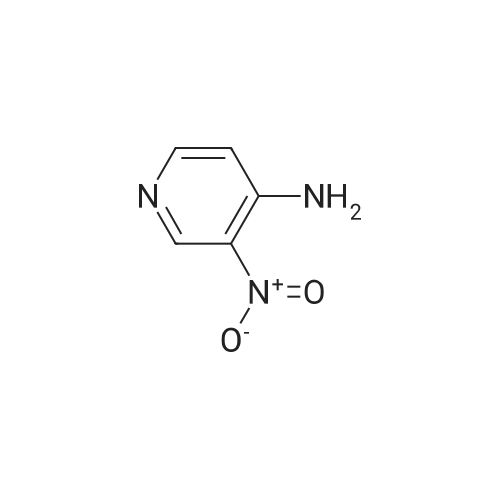
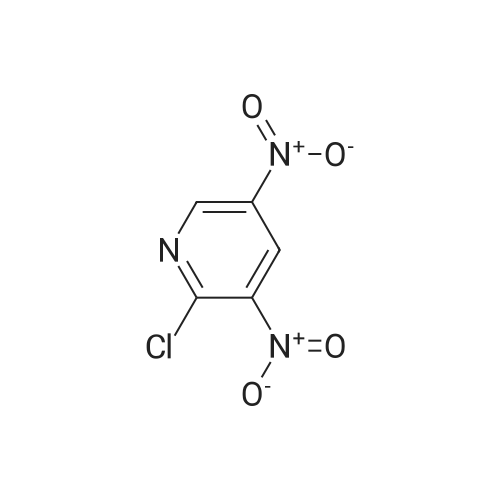
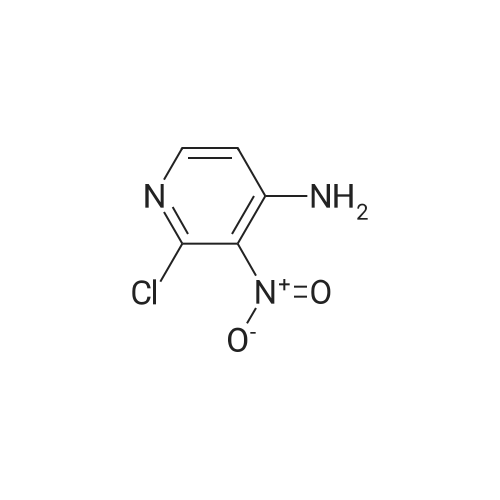
Example 136: ((2R,3S,4JR/5K)-5-{4-[(lS)-2,3-dihydro-lH-inden-l-ylamino]-lH-imidazo[4,5- c]pyridin-l-yl}-3,4-dihydroxytetrahydrofuran-2-yl)memyl sulfamate (1-149)Step a: 4-amino-2-chloro-3-nitropyridine; [0929] To a solution of 4-amino-2-chloropyridine (10.0 g, 77.8 mmol) in concentratedH2SO4 (6OmL) at 00C was added 90% nitric acid (30 mL) dropwise. The solution was stirred at 0-5 0C for 30 min then poured onto ice (carefully). The pH was brought to ~3 with concentrated aq ammonium hydroxide (~150mL) to obtain a white precipitate which was isolated and dried by filtration. The white solid was dissolved in sulfuric acid (100 mL), heated at 80 C for 5h, stirred at r.t. overnight, then poured on crushed ice. At 0 0C the pH was adjusted to ~3 with concentrated aq ammonium hydroxide (~250 mL) to obtain a yellow precipitate which was isolated by filtration. The solid was dried under vacuum overnight to obtain ~13 g of a mixture of 3- and 5-nitro isomers. A sample (4.0 g) was purified by flash chromatography (0 to 20% DCM/EtOAc) to obtain 1.77 g of the product as a fluffy yellow solid.[0930] LCMS: R.t = 1.15 min, ES+ 174 (formic acid) . |
|
With sulfuric acid; nitric acid; at 0 - 80℃;pH 1.5 - 3; |
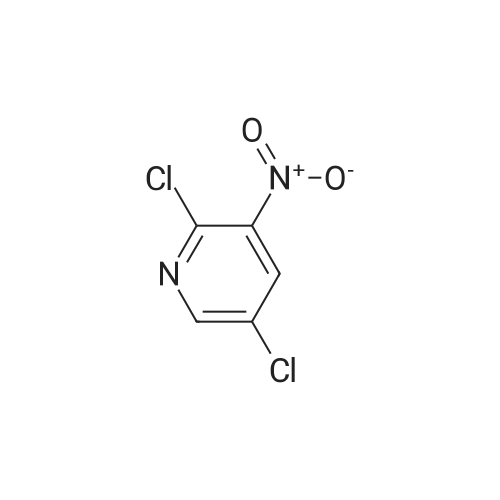
(1) at 0 C, 200 g of 2-chloro-4-aminopyridine was dissolved in 1200 mL of concentrated sulfuric acid, and 1000 mL of 65% nitric acid was added dropwise. After completion of the dropwise addition, the reaction was carried out at 15 C for 2 hours and then poured into ice water and stirred at 0 C, The NH3 was adjusted to pH 3, Precipitate a white powdery solid I, filtered. (2) The white powdery solid I was dissolved in 2000 mL of concentrated sulfuric acid, Heated to 80 C reaction 3h, stirring at room temperature overnight, and then into the ice water, 0 C into the NH3 first tune the pH to 1.5, the formation of orange precipitation, filtration, Discard the precipitate, the filtrate and then continue to NH3 to pH 3, filtration, To give a yellow powdery solid II, i.e., isomer 4-amino-2-chloro-3-nitropyridine and 4-amino-2-chloro-5-nitropyridine, the yield of the isomer was 98% Purity of 98%, among them, The yield of 4-amino-2-chloro-3-nitropyridine was 75%. Among them, the reaction at room temperature after stirring overnight there are two purposes: the first is fully responsive; the second is fully cooled; Because the reaction system concentrated sulfuric acid temperature is very high, a short period of time may not cool the internal temperature, concentrated sulfuric acid itself will be added to the water will be exothermic, if the cooling is not completely added to the ice water will be exothermic, prone to danger, so To stirred enough to cool down overnight. (3) The yellow powdery solid II was recrystallized (wherein: the recrystallization reagent was ethyl acetate and petroleum ether, the total amount was 3 times the volume of the yellow powdery solid II, the volume ratio of ethyl acetate to petroleum ether was 1:5), heated to reflux, and then reduced to 30 C to precipitate a yellow powdery solid III. The filtrate was decompressed under reduced pressure to remove the solvent. The residue was recrystallized from 95% ethanol to give the pale yellow powder The solid IV is pure 4-amino-2-chloro-5-nitropyridine in a yield of 22% and a purity of 98%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping