|
With hydrogen;palladium 10% on activated carbon; In ethanol; at 80℃; under 45004.5 Torr; |
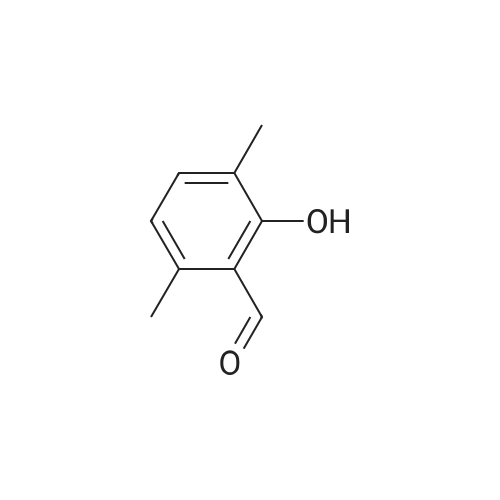
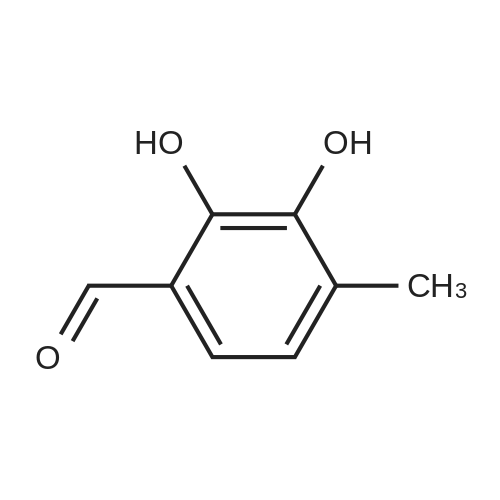
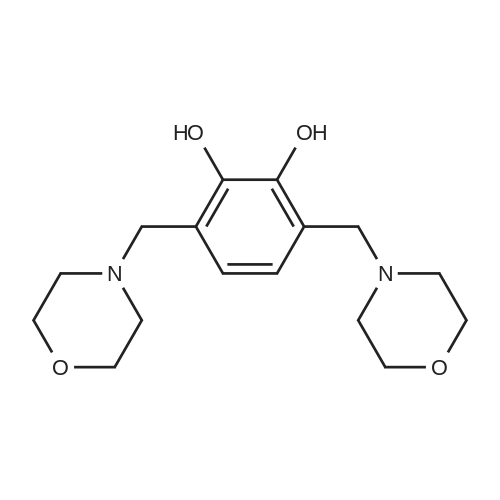
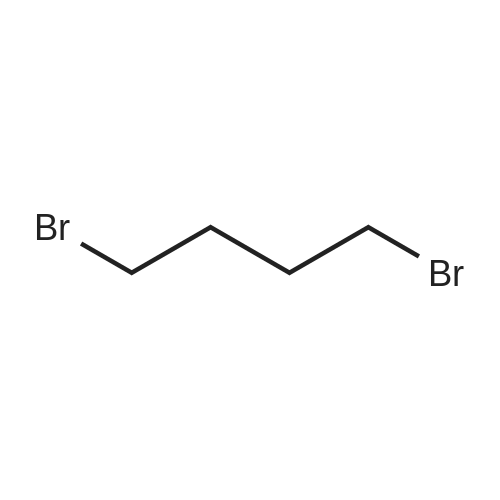
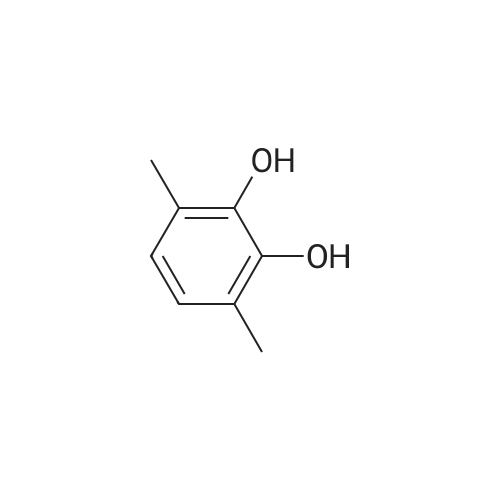
Morpholine (86 g; 0.987M) and formaldehyde (35% strength solution in water, 86 g; IM) in 250 ml of isopropanol are boiled under reflux for 10 minutes and cooled to 0 C. and a solution of 3-methylpyrocatechol (124 g; IM) in 400 ml of isopropanol is added dropwise over the course of 15 minutes. The reaction mixture is boiled under reflux for 15 minutes, cooled and concentrated in vacuo and the residue is recrystallized from isopropanol. [0358] This gives 156.5 g of the compound B2 (purity 99% by LC/MS). [0359] The compound B2 (117.44 g; 0.526M) is dissolved in 2 1 of ethanol, 48 g of 10% palladium on charcoal are added and the mixture is hydrogenated at 80 C. overnight under 60 bar of hydrogen. The hydrogenation mixture is freed from the catalyst by filtration over Kieselguhr, the filtrate is concentrated in vacuo, the residue is dissolved in chloroform and the solution is washed in succession with dilute hydrochloric acid and saturated sodium chloride solution, dried and concentrated in vacuo. [0360] This gives 34.02 g of the compound C2 (purity 85% by LC/MS). [0361] A suspension of the compound C2 (36.061 g, 0.222M) and dried, powdered potassium carbonate (108.2 g; 0.783M) in 250 ml of abs. DMF is heated to 110 C. under argon and dibromoethane (147.1 g; 0.783M) is added dropwise over 2 h. After the end of the addition, heating is continued at 110 C. for 30 minutes, the mixture is cooled and filtered with suction, the filtrate is concentrated in vacuo and the residue is dissolved in chloroform and washed 2× with dilute sodium hydroxide solution and 1× with saturated sodium chloride solution. [0362] This gives 35.7 g of the compound D2 (purity 83% by LC/MS). [0363] N-Bromosuccinimide (22.521 g; 0.1265M) is added at -30 C. to the compound D2 (22.891 g; 0.116M) in solution in 450 ml of anhydrous acetonitrile, cooling is removed and the batch is stirred overnight, filtered and concentrated in vacuo. The crude product is purified twice by column chromatography (silica gel, cyclohexane). [0364] This gives 11.6 g of the compound E2 (purity 88% by GC/MS). [0365] n-Butyllithium (15% strength in hexane, 29.58 ml; 0.048M) is added dropwise at -70 C. to the compound E2 (11.6 g, 0.042M) in 100 ml of anhydrous THF and the mixture is stirred at -70 C. for 1 h, then poured onto dry ice and left to stand overnight. Dilute sodium hydroxide solution is added to the residue, the mixture is washed 2× with ether, and acidified with concentrated hydrochloric acid, and the solid is filtered off with suction and dried. [0366] This gives 6.56 g of the compound III-2 (purity 86% by BPLC, contaminated with 12% of 5,7,8-trimethyl-1,4-benzodioxane-6-carboxylic acid), m.p. 202-3 C. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping