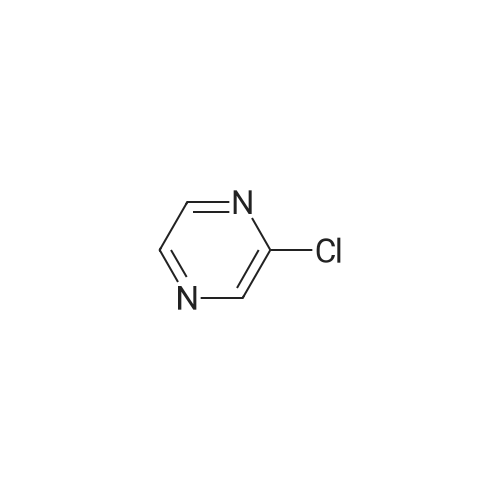
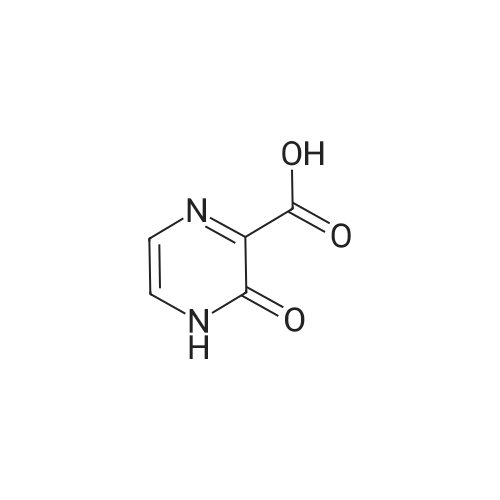
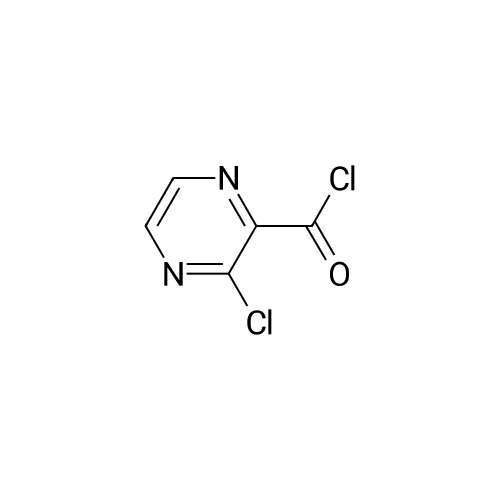
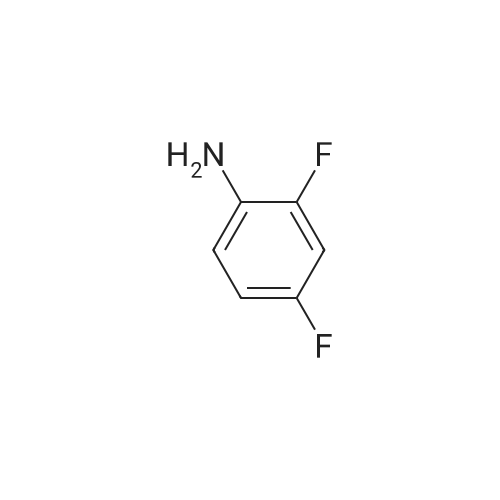
|
With thionyl chloride; N,N-dimethyl-formamide; In toluene; at 95℃; for 1h; |
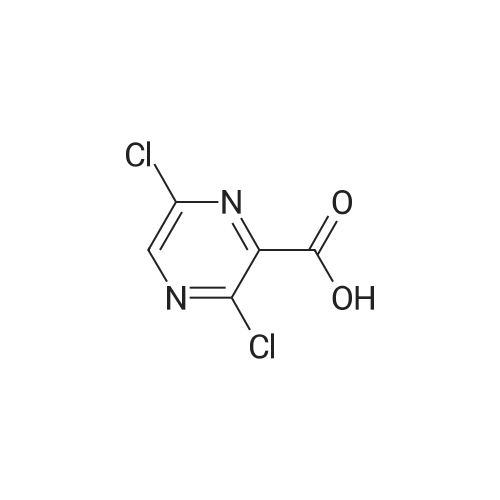
3-Cl-POA (1.0 g, 6.3 mmol) was dispersed in dry toluene (approx. 50 mL) with thionyl chloride (1.4 mL, 18.9 mmol, 3 equiv.) and a catalytic amount (1-2 drops) of N,N-dimethylformamide (DMF). The reaction mixture in round bottomed flask was stirred and heated in an oil bath under a condenser at 95 °C for approximately 1 h. Solvents were evaporated in vacuo and the residue was azeotroped with dry toluene (3 × 20 mL) to remove the unreacted SOCl2 to yield crude acyl chloride [43] as brown solid, which was used without further purification. |
|
With thionyl chloride; N,N-dimethyl-formamide; In toluene; at 95℃; for 1h; |
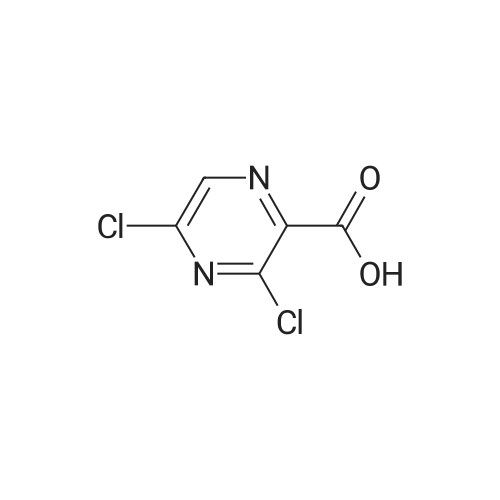
General procedure: A total of 0.3 g of 3-Cl-POA (1.9 mmol) was dispersed in dry toluene with thionyl chloride(0.4 mL, 5.7 mmol, 3 equiv.) and 1?2 drops of N,N-dimethylformamide (DMF) as a catalyst. Thereaction mixture was stirred and heated in a round bottom flask in an oil bath under a condenser,at 95°C for 1 h. The solvent was evaporated in vacuo and the residue was azeotroped with dry toluene(3 20 mL). The acyl chloride was used for the following step, without purification. The whole amount of the 3-chloropyrazine-2-carbonyl chloride prepared in the previousstep was dissolved in dry acetone. An appropriate benzylamine (5.7 mmol, 3 equiv., with respect to the starting acid), along with triethylamine (1.9 mmol, 1 equiv.), were added to thereaction mixture and stirred at laboratory temperature overnight. The progress of the reactionwas checked by TLC in system hexane/ethyl acetate (1:1 or 2:1). The reaction mixture wasadsorbed to silica by removing the solvents in vacuo and the product was purified by flashchromatography using gradient elution with ethyl acetate in hexane. For compounds 1?3,corresponding N-benzyl-3-(benzylamino)pyrazine-2-carboxamides 1a?3a were formed simultaneouslyin a molar ratio of approximately 2:3, with an excess of 1a?3a. Rf in hexane/ethyl acetate 1:1 mobilephase: 1a?0.85, 1?0.49, 2a?0.84, 2?0.51, 3a?0.71, and 3?0.40. After flash-chromatography,compounds 2, 2a, 3, 3a, 4, 8, and 13 were recrystallized from EtOH/H2O. |
|
With oxalyl dichloride; In dichloromethane; N,N-dimethyl-formamide; for 5h;Reflux; |
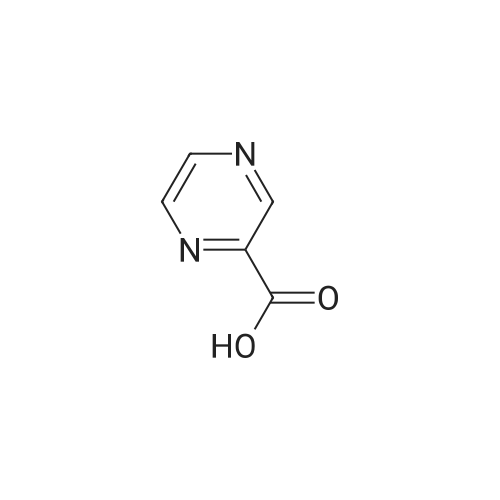
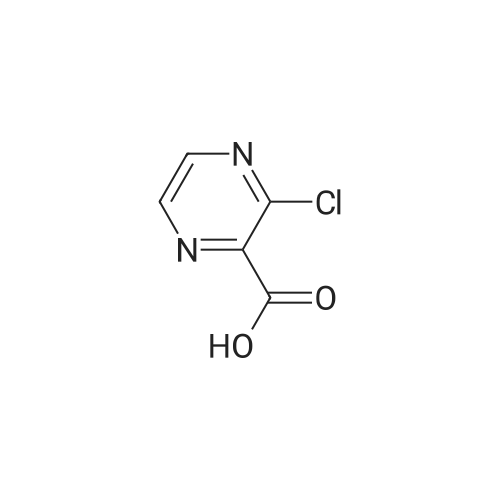
1.59 g (10 mmol) of <strong>[27398-39-6]3-chloropyrazine-2-carboxylic acid</strong> was dissolved in 20 mL of dichloromethane solvent, 2.54 g (20 mmol) of oxalyl chloride, 2 drops of DMF,After stirring at reflux for 5 h, the solvent and excess oxalyl chloride were evaporated under reduced pressure.Obtained a purplish red oil; it was dissolved in 20 mL of dichloromethane.Add 0.92g (5mmol)Ethyl 5-(methylaminomethyl)-1H-imidazole-4-carboxylate and1.52 g (15 mmol) of triethylamine,After stirring at room temperature until the reaction was completed, it was poured into 50 mL of saturated sodium bicarbonate solution, and extracted with dichloromethane (20 mL × 4).After filtration, the solvent was evaporated under reduced pressure to give ethyl 5-((3-chloro-N-methylpyrazine-2-carboxamido)methyl)-1H-imidazole-4-carboxylate.Ethyl 5-((3-chloro-N-methylpyrazine-2-carboxamido)methyl)-1H-imidazole-4-carboxylate was dissolved in 20 mL of acetonitrile.Add 3.26 g (10 mmol) of cesium carbonate,The mixture was stirred under reflux until the reaction was completed, and the solvent was evaporated under reduced pressure.It was dissolved in 20 mL of dichloromethane.Wash with distilled water (30 mL × 2), dry over anhydrous sodium sulfate,After filtration, the solvent was evaporated under reduced pressure.Column chromatography (EtOAc, 1percent Et3N),A white solid was obtained in a two step yield of 55percent. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping



-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-7-carboxamide as a PET Imaging Ligand for Metabotropic Glutamate Recept.png)