| 86% |
With 2,6-dimethylpyridine; In dichloromethane; at 0℃; for 2h; |
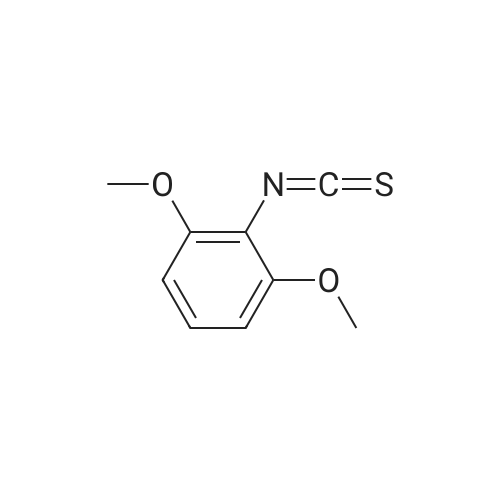
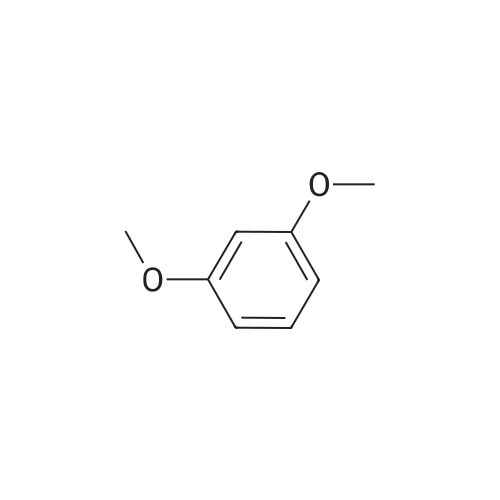
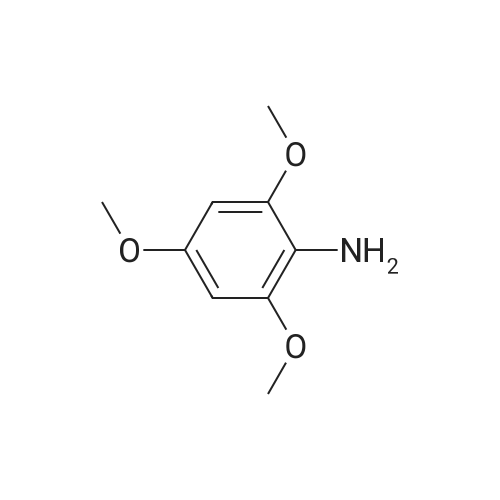
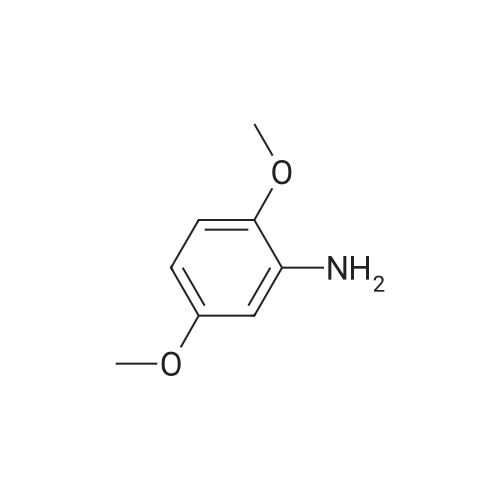
To a solution of <strong>[2734-70-5]2,6-dimethoxyaniline</strong> (500 g, 3.25 mol, 1 eq) in DCM (5.0 L) was added 2,6- lutidine (1.5 L, 13.0 mol, 4 eq). The reaction mixture was cooled to 0 C (internal temperature) and CSCl2(374 mL, 4.88 mol, 1.5 eq) was added dropwise. The reaction mixture was then stirred for 2 h. The solvent was evaporated under reduced pressure, and the material thus obtained was purified by silica column to provide the title compound, 2- isothiocyanato-1,3-dimethoxybenzene, Example 465.0 as a white solid (1.06g, 2.80 mol, 86%).1H NMR (400 MHz, CDCl3) δ 7.16 (t, J = 8.48 Hz, 1H), 6.55 (d, J = 8.48 Hz, 2H), 3.90 (m, 6H). LCMS (ESI pos. ion) m/z: (M+H)+196. |
| 86% |
With 2,6-dimethylpyridine; In dichloromethane; at 0℃; for 2h; |
To a solution of <strong>[2734-70-5]2,6-dimethoxyaniline</strong> (500 g, 3.25 mol, 1 eq) in DCM (5.0 L) was added 2,6-lutidine (1.5 L, 13.0 mol, 4 eq). The reaction mixture was cooled to 0 C (internal temperature) and CSCl2 (374 mL, 4.88 mol, 1.5 eq) was added drop-wise. The reaction mixture was allowed to stir for 2 h. The solvent was evaporated under reduced pressure and the initial product was purified by SiO2 column to provide the title compound, 2-isothiocyanato-1,3- dimethoxybenzene, Example 1.2 as white solid (1.06g, 2.80 mol, 86%). LCMS (ESI pos. ion) m/z: (M+H)+ = 196. 1H NMR (400 MHz, CDCl3) δ 7.16 (t, J = 8.48 Hz, 1H), 6.55 (d, J = 8.48 Hz, 2H), 3.90 (m, 6H). |
| 86% |
With 2,6-dimethylpyridine; In dichloromethane; at 0℃; for 2h; |
To a solution of <strong>[2734-70-5]2,6-dimethoxyaniline</strong> (500 g, 3.25 mol, 1 eq) in DCM (5.0 L) was added 2,6-lutidine (1.5 L, 13.0 mol, 4 eq). The reaction mixture was cooled to 0 C (internal temperature) and CSC12 (374 mL, 4.88 mol, 1.5 eq) was added dropwise. The reaction mixture was then stirred for 2 h. The solvent was evaporated under reduced pressure and the material thus obtained was purified by SiC column to provide the title compound, 2-isothiocyanato-l,3-dimethoxybenzene, Example 82.0 as a white solid (1.06g, 2.80 mol, 86%). LCMS (ESI pos. ion) m/z: (M+H)+ = 196. NMR (400 MHz, CDCI3) δ 7.16 (t, J= 8.48 Hz, 1H), 6.55 (d, J = 8.48 Hz, 2H), 3.90 (m, 6H). |
| 86% |
With 2,6-dimethylpyridine; In dichloromethane; at 0℃; for 2h;Inert atmosphere; |
To a solution of <strong>[2734-70-5]2,6-dimethoxyaniline</strong> (500 g, 3.25 mol, 1 eq) in DCM (5.0 L) was added 2,6- lutidine (1.5 L, 13.0 mol, 4 eq). The reaction mixture was cooled to 0 C (internal temperature) and CSC12 (374 mL, 4.88 mol, 1.5 eq) was added dropwise. The reaction mixture was then stirred for 2 h. The solvent was evaporated in vacuo and the initial mass was purified by Si02 column to provide the title compound, Example 372.0, as a white solid (1.06g, 2.80 mol, 86%). LCMS (ESI pos. ion) m/z: (M+1)+ = 196. 1HNMR (400 MHz, CDC13) ? 7.16 (t,J= 8.48 Hz, 1H), 6.55 (d,J= 8.48 Hz, 2H), 3.90 (m, 6H). |
| 53% |
With sodium hydrogencarbonate; In dichloromethane; water; at 0 - 20℃; for 1.16667h; |
To a mixture of dichloromethane (50 mL) and water (50 mL) were added 2,6- dimethoxyaniline (4.6 g, 30 mmol, 1 equiv) and sodium bicarbonate (5.0 g, 60 mmol, 2 equiv). Then thiophosgene (2.6 mL, 33 mmol, 1.1 equiv) was added in small portions with stirring at 0C. After addition, the mixture was stirred for 10 mins at 0C and then at room temperature for 1 hour. The organic layer was separated and the aqueous layer was extracted with DCM (2*50 mL). The combined organic extracts were washed with brine (30 mL), dried over anhydrous Na2S04, filtered and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (eluted with PE/EtOAc = 200/1) to afford the title compound 2-isothiocyanato-l,3-dimethoxybenzene as an off-white solid (3.1 g, 53% yield). NMR (400 MHz, DMSO-de) d: 7.29 (t, J = 8.0 Hz, 1H), 6.76 (d, J = 8.0 Hz, 2H), 3.86 (s, 6H). LC-MS: m/z 196.1 (M+H)+ |
|
With 2,6-dimethylpyridine; In dichloromethane; at 0℃; for 2h; |
To a solution of <strong>[2734-70-5]2,6-dimethoxyaniline</strong> (500 g, 3.25 mol, 1 eq) in DCM (5.0 L) was added 2,6- lutidine (1.5 L, 13.0 mol, 4 eq). The reaction mixture was cooled to 0 C (internal temperature) and CSC12 (374 mL, 4.88 mol, 1.5 eq) was added drop-wise. The reaction mixture was allowed to stir for 2 h. The solvent was then evaporated in vacuo, and the initial mass was purified by Si02 column to provide 2-isothiocyanato-1,3- dimethoxybenzene, Example 28.0 as white solid. LCMS-ESI (pos.) m/z: (M+H) = 196. 1H NMR (400 MHz, CDC13) ? 7.16 (t, J= 8.48 Hz, 1H), 6.55 (d, J= 8.48 Hz, 2H), 3.90 (app s, 6H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping