| 73% |
With iodine; potassium carbonate; In N,N-dimethyl-formamide; at 20.0℃; for 3.0h; |
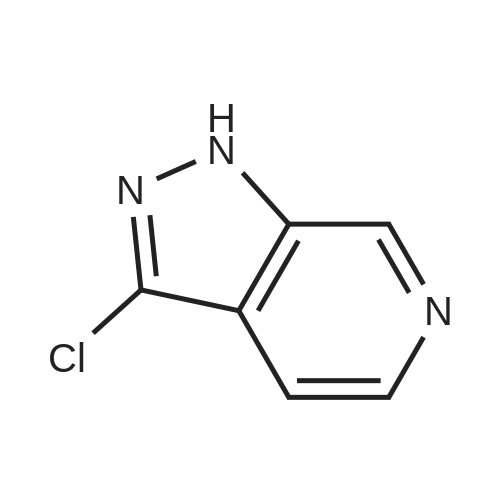
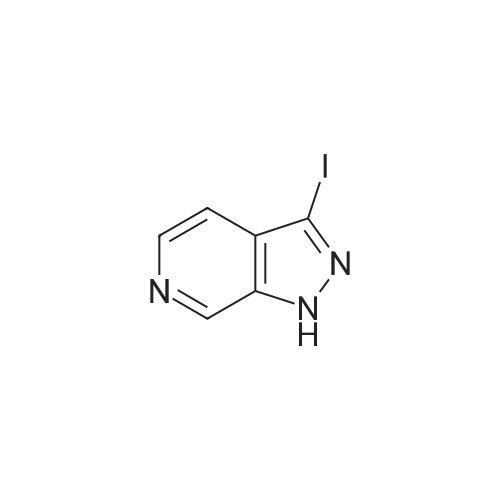
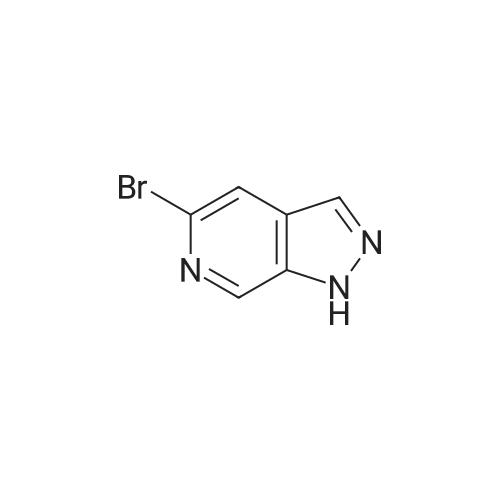
To a solution of lH-pyrazolo[3,4-c]pyridine (4.0 g, 33.6 mmol, 1.0 eq.) in DMF (40 mL) were added K2C03 (9.3 g, 100.8 mmol, 3.0 eq.), I2 (7.9 g, 33.6 mmol, 1.0 eq.). The resulting mixture was stirred at r.t. for 3 hr, then diluted by H20 and filtered. The collected solid was dried to give 3-iodo-lH- pyrazolo[3,4-c]pyridine (6.0 g, 73.0 % yield). |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h; |
To a solution of 1 H-pyrazolo[3,4-c]pyridine [271-47-6 ] (4.00 g, 33.6 mmol) in DMF (50 mL) were added iodine (12.8 g, 50.4 mmol) and potassium hydroxide (4.70 g, 84.0 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was diluted with 10% sodium thiosulfate and water, then extracted (3x) with EtOAc. The combined organic extracts were washed with brine, then dried (Phase separator) and concentrated under vacuum. MS (LC/MS): 246.0 [M+H]+; tR (HPLC conditions k): 0.48 min. |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h; |
A. 3-Iodo-I H-pyrazolo[3,4-c]pyridineTo a solution of 1 H-pyrazolo[3,4-c]pyridine [27 1-47-6 1(4.00 g, 33.6 mmol) in DMF (50 mL) were added iodine (12.8 g, 50.4 mmol) and potassium hydroxide (4.70 g, 84.0 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was diluted with 10% sodium thiosulfate and water, then extracted (3x) with EtOAc. The combined organic extracts were washed with brine, dried (Phase separator) and concentrated under vacuum. MS (LCMS): 246.0 [M+H]+; tR (HPLC conditions d): 0.48 mm. |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h; |
A. 3-lodo-1 H-pyrazolo[3,4-c]pyridineTo a solution of IH-pyrazolo[3,4-c]pyridine [271-47-6 ] (4.00 g, 33.6 mmol) in DMF (50 mL) were added iodine (12.8 g, 50.4 mmol) and potassium hydroxide (4.70 g, 84.0 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was diluted with 10% sodium thiosulfate and water, then extracted (3x) with EtOAc. The combined organic extracts were washed with brine, dried (phase separator) and concentrated. MS (LC/MS): 246.0 [M+H]+; tR (H PLC conditions d): 0.48 mm. |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h; |
To a solution of 1 H-pyrazolo[3,4-c]pyridine [271-47-6 ] (4.00 g, 33.6 mmol) in DMF (50 mL) were added iodine (12.8 g, 50.4 mmol) and potassium hydroxide (4.70 g, 84.0 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was diluted with 10% sodium thiosulfate and water, then extracted (3x) with EtOAc. The combined organic extracts were washed with brine, dried (Phase separator) and concentrated under vacuum. MS (LC/MS): 246.0 [M+H]+; tR (HPLC conditions d): 0.48 min. |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h; |
To a solution of 1H-pyrazolo[3,4-c]pyridine [271-47-6] (4.00 g, 33.6 mmol) in DMF (50 mL) were added iodine (12.8 g, 50.4 mmol) and potassium hydroxide (4.70 g, 84.0 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was diluted with 10% sodium thiosulfate and water and extracted with EtOAc (3x). The combined organic extracts were washed with brine, dried (phase separator) and concentrated to afford the title compound. MS (LCMS): 246.0 [M+H]+; tR (H PLC conditions d): 0.48 mm. |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h;Inert atmosphere; |
To a solution of 1H-pyrazolo[3,4-c]pyridine [271-47-6 1(4.00 g, 33.6 mmol) in DMF (50 mL) were added iodine (12.8 g, 50.4 mmol) and potassium hydroxide (4.70 g, 84.0 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was diluted with 10% sodium thiosulfate and water, then extracted (3x) with EtOAc. The combined organic extracts were washed with brine, dried (Phase separator) and concentrated under vacuum. MS (LCMS):246.0 [M+H]+; tR (H PLC conditions b): 0.48 mm. |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h; |
To a solution of 1H-pyrazolo[3,4-c]pyridine [271-47-61(4.00 g, 33.6 mmol) in DMF (50 mL) were added iodine (12.8 g, 50.4 mmol) and potassium hydroxide (4.70 g, 84.0 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was diluted with 10% sodium thiosulfate and water, then extracted with EtOAc (3x). The combined organic extracts were washed with brine, dried (phase separator) and concentrated. MS (LCMS): 246.0 [M+H]+; tR (H PLC conditions d):0.48 mm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping