|
|
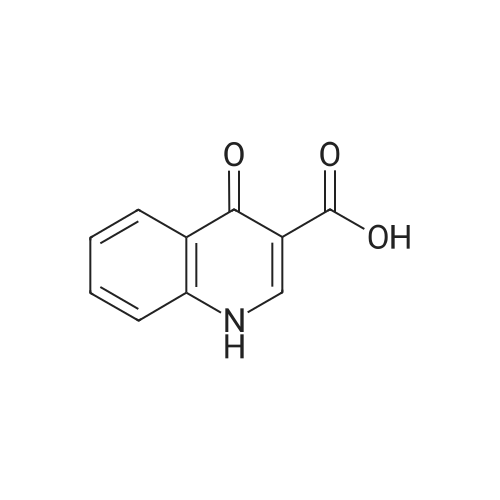
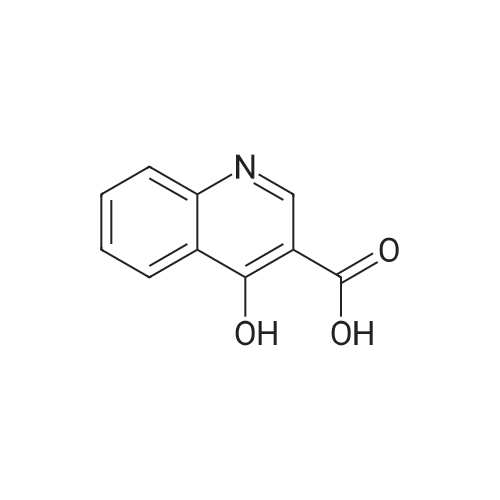
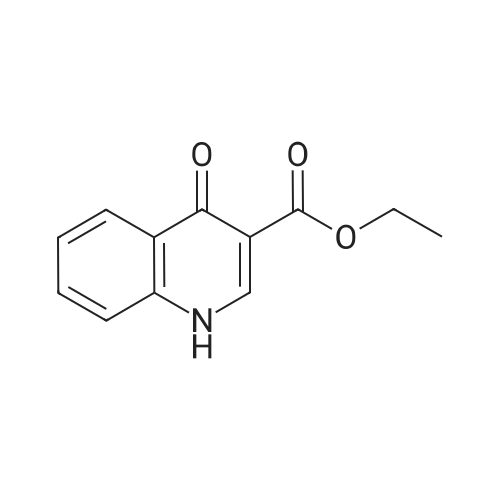
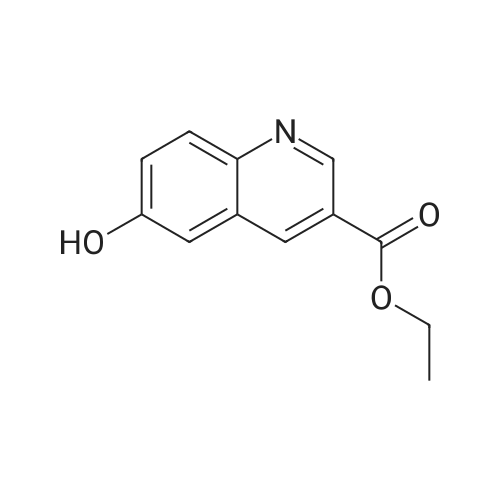
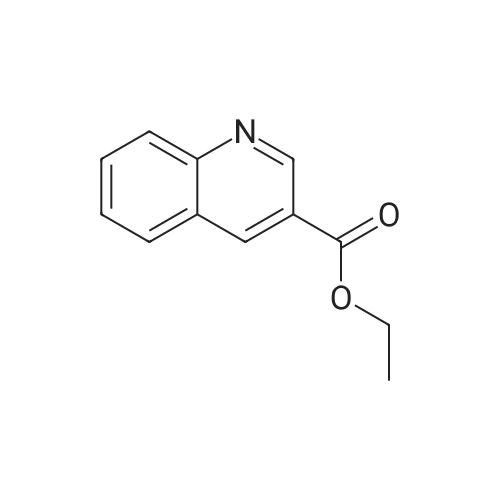
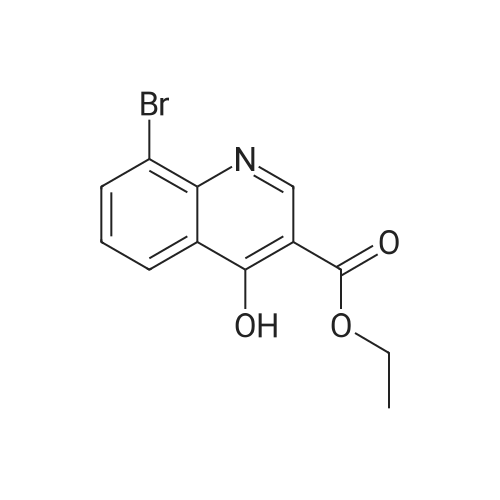
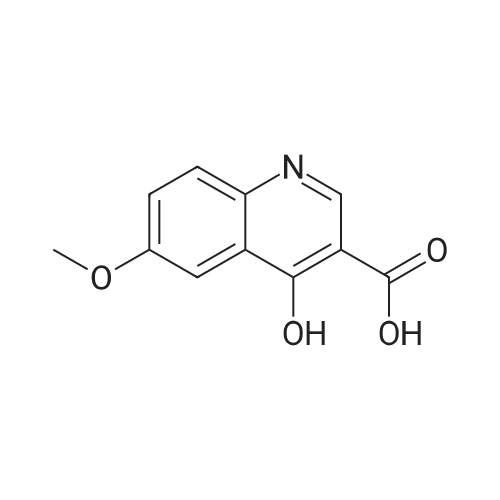
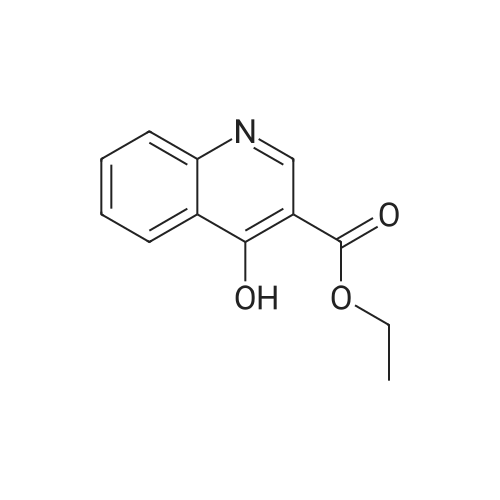
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h under reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HCl. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-1,4-dihydroquinoline-3-carboxylic acid (A-1) as a pale white solid (10.5 g, 92%). 1H NMR (d-DMSO) delta 15.34 (s, 1H), 13.42 (s, 1H), 8.89 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J=8.4 Hz, 1H), 7.60 (m, 1H). |
|
With sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxidesolution (2N, 150 mL) and stirred for 2 h under reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HCl. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-1,4-dihydroquinoline-3-carboxylic acid (A-1) as a pale white solid (10.5 g, 92%).1H NMR (d-DMSO) delta 15.34 (s, 1H), 13.42 (s, 1H), 8.89 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J=8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HCl. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-l,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %). 1H NMR (DMSO- |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HCl. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-1,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %).1H NMR (DMSO-d6) delta 15.34 (s, 1 H), 13.42 (s, 1 H), 8.89 (s, 1H), 8.28 (d, J = 8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
Step C: 4-Oxo-1,4-dihydroquinoline-3-carboxylic acid 4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in a sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HCl. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-1,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92%). 1H NMR (DMSO-d6) delta 15.34 (s, 1H), 13.42 (s, 1H), 8.89 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J=8.4 Hz, 1H), 7.60 (m, 1H) ppm. |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
j00332J 4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HC1. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-1,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %). ?H NIVIR (DMSO-d6) 15.34 (s, 1 H), 13.42 (s, 1 H), 8.89 (s, 1H), 8.28 (d, J= 8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HC1. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-l,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %). lU NMR (DMSO-4) delta 15.34 (s, 1H), 13.42 (s, 1H), 8.89 (s, 1H), 8.28 (d, = 8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HCl. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-l,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %). lU NMR (DMSC fc) delta 15.34 (s, 1H), 13.42 (s, 1H), 8.89 (s, 1H), 8.28 (d, 7 = 8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With water; sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HC1. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-l,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %). lU NMR (DMSO-4) delta 15.34 (s, 1 H), 13.42 (s, 1 H), 8.89 (s, 1H), 8.28 (d, J = 8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3 -carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HC1. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-l,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %). 1H NMR (DMSO-ifc) d 15.34 (s, 1 H), 13.42 (s, 1 H), 8.89 (s, 1H), 8.28 (d, 7 = 8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With sodium hydroxide; for 2h;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HC1. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-1,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92 %). 1H NMR (DMSO-rfc) d 15.34 (s, 1H), 13.42 (s, 1H), 8.89 (s, 1H), 8.28 (d, 7 = 8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.60 (m, 1H). |
| 10.5 g |
With water; sodium hydroxide; at 2℃;Reflux; |
4-Hydroxyquinoline-3-carboxylic acid ethyl ester (15 g, 69 mmol) was suspended in sodium hydroxide solution (2N, 150 mL) and stirred for 2 h at reflux. After cooling, the mixture was filtered, and the filtrate was acidified to pH 4 with 2N HCl. The resulting precipitate was collected via filtration, washed with water and dried under vacuum to give 4-oxo-1,4-dihydroquinoline-3-carboxylic acid as a pale white solid (10.5 g, 92%). 1H NMR (DMSO-d6) delta 15.34 (s, 1H), 13.42 (s, 1H), 8.89 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 7.88 (m, 1H), 7.81 (d, J=8.4 Hz, 1H), 7.60 (m, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping