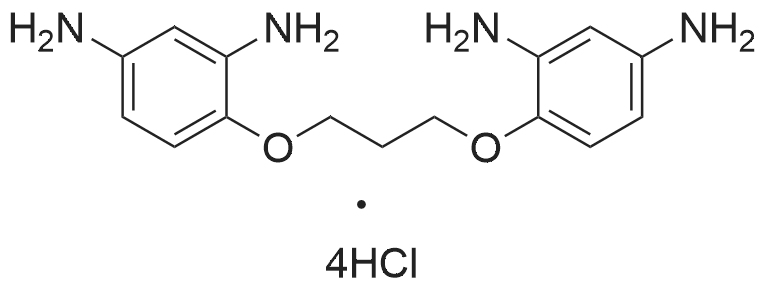
| 56.5% |
|
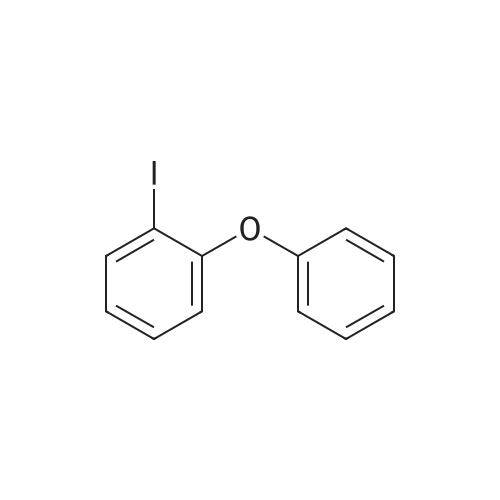
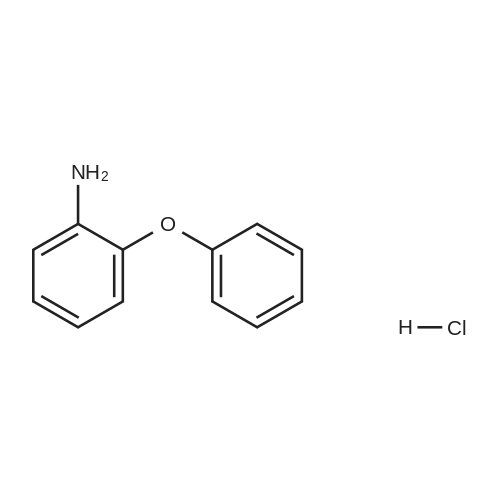
1L into a round bottom flask 2-phenoxyaniline (25.0, 0.135mol) and hydrochloric acid, 30ml, 150ml water, cooled to 0 degrees and the mixture was stirred for 1 hour. After the addition the reaction mixture of sodium (11.2g, 0.162mol) in 75ml aqueous solution of the same temperature and the mixture was stirred for one hour. Potassium iodide (44.8g, 0.270mol) and note the temperature of the reaction solution was added dropwise not exceed 5 was added dropwise to 75ml of an aqueous solution. Stirred for 5 hours at room temperature after the completion of the reaction haejugo After washing with sodium sayi oh sulfate aqueous solution was extracted with ethyl acetate and water. The organic layer was separated and concentrated under reduced pressure and purified by column chromatography to obtain a intermediate 5-a> (22.6g, 56.5%) |
| 56.5% |
|
1L round bottom flask reactor2-phenoxy aniline (25.0, 0.135mol)And hydrochloric acid 30ml,Add water to 150ml and cooled to 0 degreesIt was stirred for 1 hour. After dropping a sodium nitro discrete (11.2g, 0.162mol) in 75ml aqueous solution even on the same it was stirred for 1 hour.Potassium iodide (44.8g, 0.270mol)The temperature of the reaction solution, 75ml of an aqueous solution Do not exceed 5 and added dropwise. After stirring for 5 hours at room temperature haejugo complete reaction after washing with sodium thiosulfate aqueous solution with ethyl acetate and waterIt was extracted. The organic layer was concentrated under reduced pressure and separated by column chromatography to give the (22.6g, 56.5%). |
| 56.5% |
|
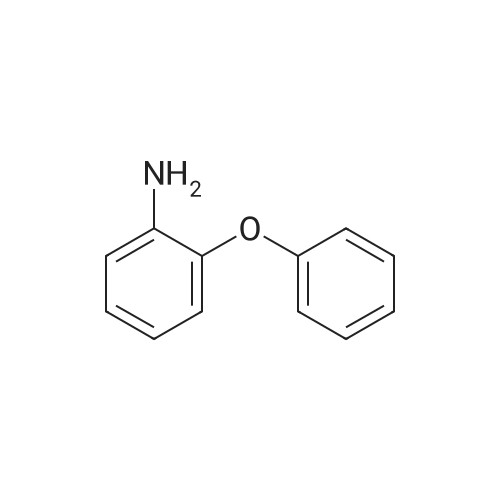
In a 1 L round bottom flask reactor 2-Phenoxyaniline (25.0, 0.135 mol), hydrochloric acid (30 ml) and water (150 ml) were added and the mixture was cooled to 0 C and stirred for 1 hour. 75 ml of an aqueous solution of sodium nitrite (11.2 g, 0.162 mol) was added dropwise to the reaction solution at the same temperature, followed by stirring for 1 hour. When 75 ml of an aqueous solution of potassium iodide (44.8 g, 0.270 mol) was added dropwise, the temperature of the reaction solution was dropped so that the temperature did not exceed 5 C. After stirring for 5 hours at room temperature, the reaction mixture was washed with an aqueous solution of sodium cyanosulfate and extracted with ethyl acetate and water. The organic layer was separated and concentrated under reduced pressure, followed by separation and purification by column chromatography to obtain Intermediate 5-a. (22.6 g, 56.5%). |
| 56.5% |
|
2-phenoxyaniline (25.0, 0.135 mol) and 30 ml of hydrochloric acid were added to a 1 L round bottom flask reactor, and the mixture was cooled to 0 C and stirred for 1 hour.75 ml of an aqueous solution of sodium nitrite (11.2 g, 0.162 mol) was added dropwise to the reaction solution at the same temperature, followed by stirring for 1 hour.When the aqueous solution of potassium iodide (44.8 g, 0.270 mol) was added dropwise, the temperature of the reaction solution was prevented from exceeding 5 and dropping.The mixture was stirred at room temperature for 5 hours and washed with an aqueous sodium thiosulfate solution after completion of the reaction, followed by extraction with ethyl acetate and water.The organic layer was separated and concentrated under reduced pressure, and then purified by column chromatography to obtain Intermediate 5-a (22.6 g, 56.5%). |
| 56.5% |
|
In a 1 E round bottom flask reactor, a mixture of 2 phenoxyaniline (25.0, 0.135 mol), HC1 (30 ml), and water (150 ml) was cooled to 0 C. and stirred for 1 hr. At the same temperature, an aqueous solution (75 ml) of sodium nitrite (11.2 g, 0.162 mol) was added and then stirred for 1 hr. An aqueous solution (75 ml) of potassium iodide (44.8 g, 0.270 mol) was dropwise added, taking care not to increase the temperature of the reaction solution above 5 C. Stirring was continued for 5 irs at room temperature, and after completion of the reaction, the reaction mixture was washed with an aqueous sodium thiosulfate solution and extracted with ethyl acetate and watet The organic layer was separated and concentrated in a vacuum. Purification through colunm chromatography gave Intermediate 5-a (22.6 g, 56.5%). |
| 56.5% |
|
According to Scheme 34, intermediate 5-a was synthesized:2-Penoxyaniline (25.0, 0.135 mol), 30 ml of hydrochloric acid and 150 ml of water were added to a 1 L round bottom flask reactor, and the mixture was cooled to 0 C and stirred for 1 hour.At the same temperature, 75 ml of an aqueous solution of sodium nitride (11.2 g, 0.162 mol) was added dropwise, followed by stirring for 1 hour.75 ml of an aqueous solution of potassium iodide (44.8 g, 0.270 mol) was added dropwise noting the temperature of the reaction solution not to exceed 5 degrees. After stirring for 5 hours at room temperature, the reaction was completed, washed with sodium thiosulfate aqueous solution and extracted with ethyl acetate and water.The organic layer was concentrated under reduced pressure and separated by column chromatography to obtain <Intermediate 5-a (22.6 g, 56.5%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping