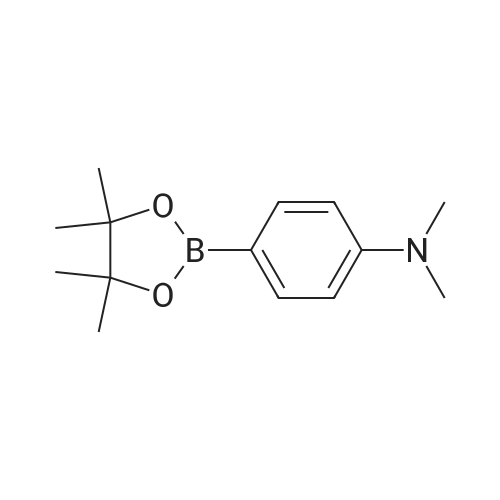
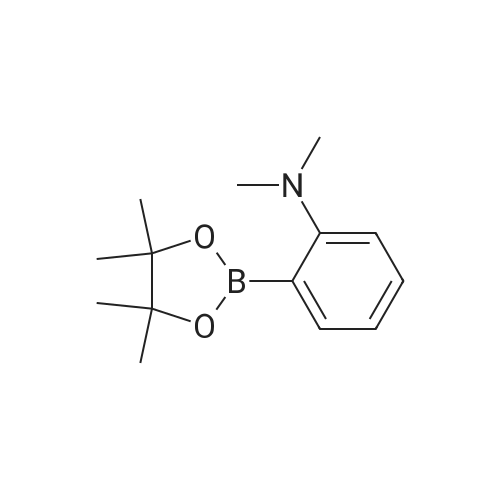
| 89% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 90℃; |
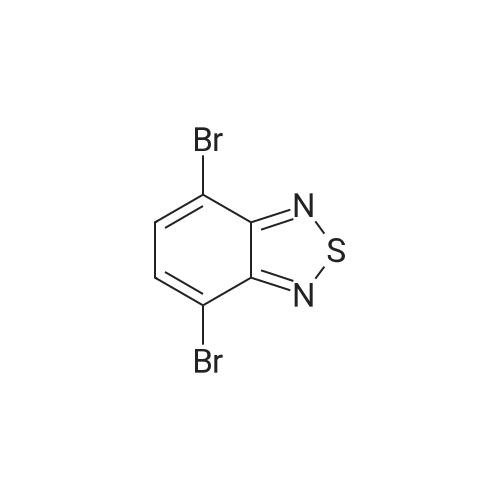
Said M 1-I-1 obtained in the synthesis (112.78g, 347.9mmol) senses a rotation velocity of the disk to a DMF to in round bottom flask , Bis (pinacolato) diboron (97.17g, 382.6mmol), Pd (dppf) Cl 2 (8.52g, 10.4mmol), KOAc (102.42g, 1043.6mmol) added 90 C stirring section. When reaction is completed by etching are removed and then the DMF via fractional distillation CH 2 Cl 2 extracted and water. Organic layer MgSO 4 to dry a silicagel column with a compound formed after the products and recrystallization 114.95g (yield: 89%)is obtained. |
| 89% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In dimethyl sulfoxide; at 80℃; for 6h; |
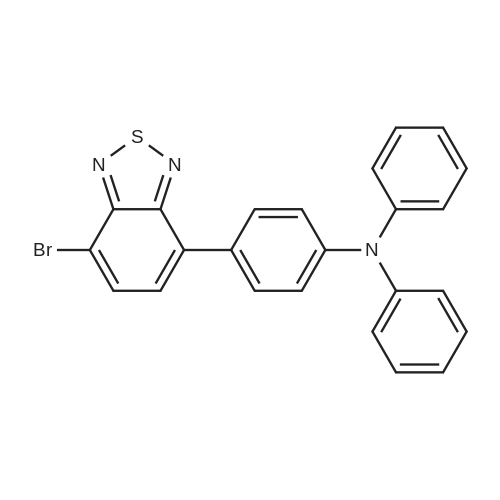
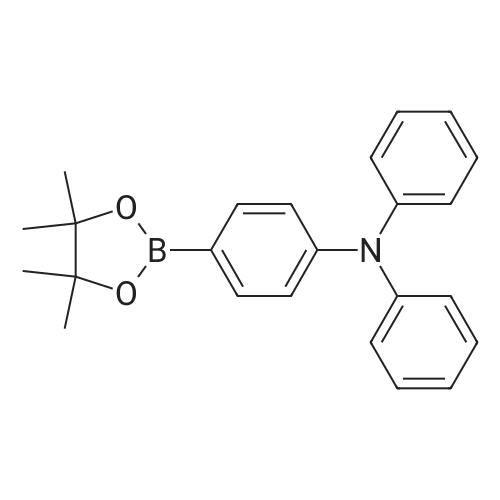
4-bromo-triphenyl amine 3.24g (10.0mmol), Bis (pinacolato) diborone 2.54g (10.0mmol), PdCl2 (dppf) 2 0.36g (0.5mmol), and KOAc 2.94g (30.0mmol) dissolved in 40mL of DMSO after stirring at 80C for 6 hours. After cooling the reaction solution to room temperature and extracted three times with 50mL of water and 50mL of diethyl ether. Dry the organic layer obtained therefrom by separating the magnesium sulfate, and the residue obtained by evaporating the solvent was subjected to silica gel column chromatography to give the purified intermediate I-8 2.57g (89% yield). The resulting compound was confirmed by NMR and HR-MS. |
| 89% |
With 1,1'-bis-(diphenylphosphino)ferrocene; potassium acetate; In 1,4-dioxane; for 21h;Inert atmosphere; Reflux; |
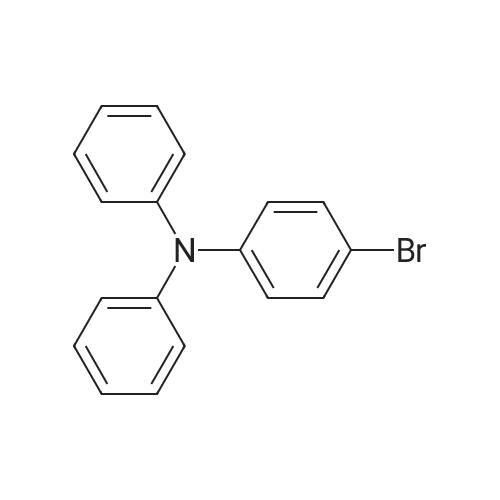
Under an argon atmosphere, palladium chloride (80 mg, 0.45 mmol) and 1,1'-bis(diphenylphosphino)ferrocene (249 mg, 0.45 mmol) were dissolved in 1,4-dioxane (40 mL) After stirring, bromotriphenylamine (4.86 g, 15.0 mmol), bispinacolatodiboron (4.19 g, 16.5 mmol), potassium acetate (4.42 g, 45.0 mmol) and dioxane (90 mL) were added to a solution of Was added sequentially. The reaction mixture was stirred under heating reflux for 21 hours. After allowing to cool to room temperature, water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. Sodium sulfate was added to the organic layer, which was separated by filtration, and the solvent was distilled off to obtain a crude product. This was purified by silica gel column chromatography (developing solvent: hexane / chloroform = 2/1 to 1/1) to obtain the objective 4-(diphenylamino)phenylboronic acid pinacol ester as a white solid (4.96 g , 89%). |
| 89.4% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 85℃; for 12h;Inert atmosphere; |
4-Bromotriphenylamine (5.01 g, 15.43 mmol), bis(pinacolato)diboron (9.81 g, 38.58 mmol), PdCl2(dppf) (1.13 g, 1.54 mmol), KOAc (6.05 g, 61.72 mmol) in a 100 ml three-necked flask, the air in the reaction tube was replaced with N2, and 50 ml of 1,4-dioxane was injected as a solvent under N2, and reacted at 85 C for 12 h. After completion of the reaction, the mixture was cooled to room temperature. After cooling, the reaction mixture was evaporated to dryness, purification by silica gel column chromatography (V ( petroleum ether) / V (dichloromethane) = 100/1) to obtain white solid M-1 (5.1 g, 89.4%). |
| 88.1% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In tetrahydrofuran; at 80℃; for 5h;Inert atmosphere; |
In a 250 mL three-necked flask, nitrogen was bubbled with 0.02 mol of 4-bromotriphenylamine in 100 ml of tetrahydrofuran (THF) and 0.024 mol of bis (pinacolato) diboron, 0.0002 mol (Diphenylphosphino) ferrocene) dichloropalladium (II) and 0.05 mol of potassium acetate were added, and the mixture was stirred, and the mixed solution of the above reaction was heated under reflux at reflux temperature for 5 hours. After the completion of the reaction , Cooled and added with 100 ml of water, and the mixture was filtered and dried in a vacuum oven. The obtained residue was purified by silica gel column to obtain triphenylamine-4-boronic acid pinacol ester; HPLC purity was 99.7% and the yield was 88.1%. |
| 87% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 90℃; |
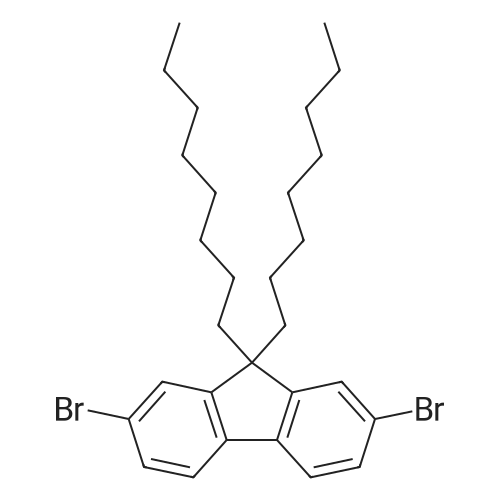
Wherein M 2-I-1 (63.01 g, 194.3 mmol) to a round bottom flask was charged with DMF (970 ml) to dissolve later, Bis (pinacolato) diboron (54.29 g, 213.8 mmol), PdCl2 (dppf) (4.76 g, 5.8 mmol), was added KOAc (57.22 g, 583 mmol) and the resulting mixture was stirred at 90 C. After completion of reaction by distillation to remove DMF and CH2Cl2. And extracted with water. The organic layer was dried over MgSO4 and concentrated and the resulting product was compound silicagel column and recrystallization 62.78 g (yield:87% was obtained). |
| 86% |
With 1,1'-bis-(diphenylphosphino)ferrocene; potassium acetate; palladium diacetate; acetic acid; In 1,4-dioxane; at 90℃; for 15h;Inert atmosphere; Reflux; |
To a two-necked flask was added Compound 3 (4.86 g, 15 mmol), pinacolate (4.95 g, 19.5 mmol),Palladium acetate (0.1 g, 0.45 mmol), dppf (0.5 g, 0.9 mmol) and potassium acetate (4.42 g, 45 mmol)Dioxane (50 ml) was added, and the mixture was refluxed at 90 C for 15 h. After the reaction, deionized water (3x50ml) was extracted with DCM (100ml). The organic phase was dried over anhydrous magnesium sulphate and evaporated to DCM. The product 5 was obtained by column chromatography. |
| 80% |
|
To a three-necked flask adding 1,4-dioxane solution of 4-bromotriphenyl amine (5 g, 15.42 mmol), bis(pinacolato)diboron (4.7 g,18.5 mmol) and CH3COOK (4.54 g, 46.62 mmol), The solution waspurged with argon for 30 min, and then Pd(dppf)Cl2 (0.38 g,0.465 mmol) was added. The reaction was stirred at 85 C overnight.The reaction followed by TLC until reaction completion, then let the reaction to cool down and the reaction quenched by adding (50 ml) ofwater then extracted by ethyl acetate (3×30 ml) The combined organiclayers were dried over anhydrous Mg2SO4, and the solvent wasremoved under vacuum. The crude product was purified by column chromatography on silica with hexane/ethyl acetate mixture (6: 1). The compound crystalized from anhydrous ethanol and give the pure white powder. Yield (80%), melting point (m.p) 93 C. FT-IR (cm-1):2976-2990 (CH for CH3), 1591 (C = C aromatic), 1278 (C--N aromaticamine). |
| Ca. 75% |
With bis-triphenylphosphine-palladium(II) chloride; potassium acetate; In N,N-dimethyl-formamide; for 6h;Inert atmosphere; Reflux; |
Compound 2was synthesized according to the modified reportedprocedures.25 4-Bromo triphenylamine, 1 (0.32 g,1 mmol), bis(pinacolato)diborane (0.38 g, 1.5 mmol),KOAc (0.4 g, 4 mmol) and Pd(PPh3)2Cl2 (35mg,0.05 mmol) were dissolved in minimum amount ofdegassed N,N-dimethyl formamide (DMF) in a 100mLround bottom (RB) flask. Subsequently, the reactionmixture was subjected to reflux for 6 h under nitrogenatmosphere and the reaction was monitored by TLC.After completion of reaction, the solvent was evaporatedunder vacuum and crude compound was extractedwith dichloromethane and concentrated using rotaryevaporator. The crude compound was purified by columnchromatography by employing silica gel as stationaryphase and ethyl acetete:hexane (2:98) as eluting solvent.Yield ~75%. |
| 75% |
With tris-(dibenzylideneacetone)dipalladium(0); sodium acetate; tri tert-butylphosphoniumtetrafluoroborate; In 1,4-dioxane; for 12h;Inert atmosphere; Reflux; |
General procedure: Under the protection of argon, compound 4 (1 eq.), bis(pinacolate)diboron (1.25 eq.), NaOAc (3.12 eq.) were dissolved in dry argonpurged1,4-dioxane. Then, Pd2dba3 (0.005 eq.) and [(t-Bu)3PH]BF4(0.02 eq.) were added to the system and stirring at reflux for 12 h. Thereaction mixture was then cooled down to room temperature and extractedwith CH2Cl2, the organic layer was dried over anhydrousNa2SO4, and the solvent was removed by rotary evaporator. The residuemixture was purified by column chromatography on silica gel usinghexane/CH2Cl2 (1:1) as the eluent to obtain the derivatives 5a and 5b.2.5.1. (5a)Yield 75%, 93 mg. 1H NMR (400 MHz, CHCl3-d) 4/ppm=4 7.67 (d,J=8.5 Hz, 2H), 7.26 (t, J=8.0 Hz, 4H), 7.11 (d, J=7.5 Hz, 4H), 7.05(t, J=8.5 Hz, 4H), 1.34 (s, 12H). 13C NMR (100 MHz, CHCl3-d) 4/ppm=150.6, 147.4, 136.4, 135.9, 129.3, 125.0, 123.4, 121.8, 83.6,24.9. MS (EI): m/z calcd for [C24H26BNO2]+: 371.29; found: 371.40. Anal. Calcd for C24H26BNO2: C, 77.64; H, 7.06; N, 3.77%. Found: C,77.59; H, 7.02; N, 3.75%. |
| 63% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; at 85℃; for 15h;Inert atmosphere; |
Under inert atmosphere, a degassed solution of 1(3.000 g, 9.250 mmol), bis(pinacolato)diboron (3.054 g,12.255 mmol), KOAc (3.018 g, 32.225 mmol),and Pd(dppf)2Cl2 (0.338 g, 0.05 mmol) in drydimethoxyethane (30 mL) was heated under reflux conditionsfor 15 h. After this period, the mixture wascooled to room temperature, filtered, and diluted withCH2Cl2 (50 mL). The organic solution was washed withH2O (2×30 mL) and brine, then dried (with anhydrousMgSO4 and evaporated. The residue was separated bycolumn chromatography using hexane/CH2Cl2 (9/1 v/v)to give compound 2 as a white solid product (2.170 g,63%). 1H NMR (300 MHz, CDCl3: 7.74-7.59 (d, 2H),7.19-7.08 (d, 4H), 7.07-6.90 (t, 4H), 1.48-1.16 (t, 12H). |
| 63% |
With palladium bis[bis(diphenylphosphino)ferrocene] dichloride; potassium acetate; In 1,2-dimethoxyethane; for 15h;Heating; |
Substance (1) (3.000 g, 9.250 mmol), bis (pinacolao) diboron) (3.054 g, 12.255 mmol), KOAc (3.018 g,32.225 mmol) and Pd (dppf) 2 Cl 2 (0.338 g, 0.05 mmol) are heated in dried Dimethoxyehane (30 mL) for 15 h.The product is then cooled to room temperature, filtered and diluted with dichloromethane (CH 2 Cl 2, 50 mL). The prepared solution is washed with water and brine, dried over anhydrous magnesium sulfate (MgSO 4) and evaporated.The final product was then separated using hexane / dichlorometal (hexane / Ch2Cl2 (9/1 v / v) column chromatography) to give a white solid (2.170 g, yield = 63%). |
| 60% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 16h;Inert atmosphere; Reflux; |
A mixture of [1,1′ -Bis(diphenylphosphino)ferrocene]dichloropalladium(II) (0.20 g, 0.27 mmol), bis(pinacolato)diboron (2.44 g, 9.60 mmol), 4-bromo-N,N-diphenylamine (2.56g, 8.00 mmol), KOAc (2.16 g, 22.00 mmol) and dehydrated 1,4-dioxane(100 mL) in a round bottom flask was refluxed under argon atmospherefor 16 h. The reaction mixtures were concentrated, extracted bydichloromethane, and purified by column chromatography (petroleumether/dichloromethane = 10/4, v/v) to afford the white solids with ayield of 60% (1.78 g). 1H NMR (600 MHz, CDCl3) 7.66 (d, J = 8.4 Hz,2H), 7.26-7.24 (m, 4H), 7.10 (d, J = 7.8 Hz, 4H), 7.05-7.02 (m, 4H),1.33 (s, 12H). |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 85℃; for 24h;Inert atmosphere; |
(1) Add in a 250 ml three-neck round bottom reaction flask under a nitrogen atmosphere9.71 g of 4-bromotriphenylamine and 15.6 g of pinacol borate, followed by 240 ml of dioxane,24 g of potassium acetate was agitated for 30 minutes.Finally, 700 mg of [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride was added, and after aeration for 30 minutes, it was heated to 85 C and refluxed for 24 hours.After the reaction is stopped and the reaction is stopped, the solvent of the reaction system is removed by rotary evaporation.The intermediate product N,N-biphenyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline was obtained. |
| 9.2 g |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In dichloromethane; dimethyl sulfoxide; at 90℃; for 8h;Inert atmosphere; |
In a reaction vessel under a nitrogen atmosphere, 10.0 g of a compound represented by the formula (L1-1-27-3), 8.6 g of bis (pinacolato) diborone, 4.5 g of potassium acetate,100 mL of dimethyl sulfoxide and 0.5 g of [1,1'-bis (diphenylphosphino) ferrocene] palladium (II) dichloride dichloromethane adduct were added, and the mixture was heated with stirring at 90 C for 8 hours. The reaction solution was poured into water and extracted with ethyl acetate. The organic layer was washed successively with water and brine. Purification by column chromatography (alumina, ethyl acetate) gave 9.2 g of a compound represented by the formula (L1-1-27-4). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping