| 65% |
|
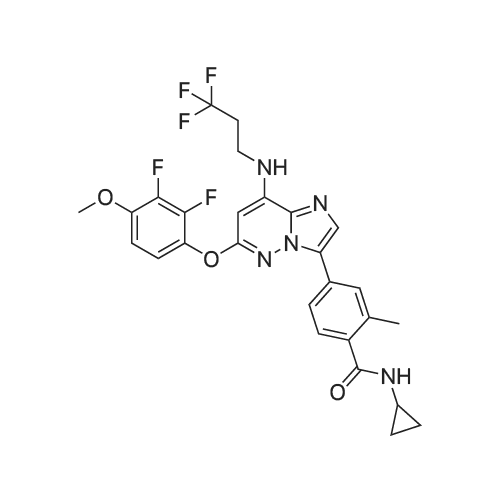
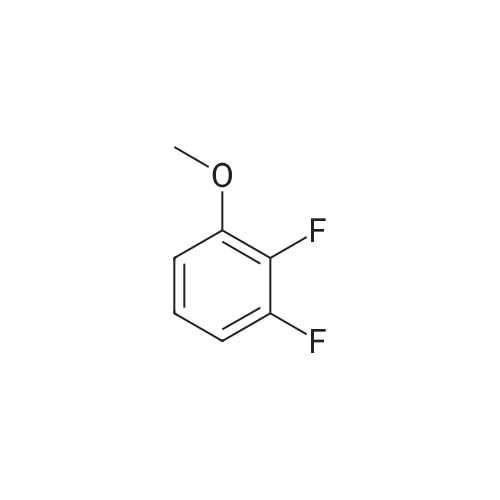
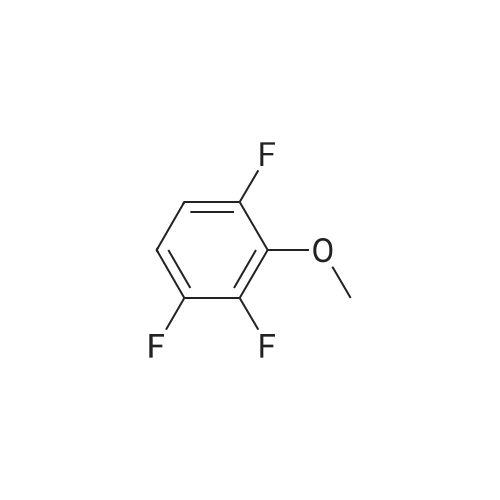
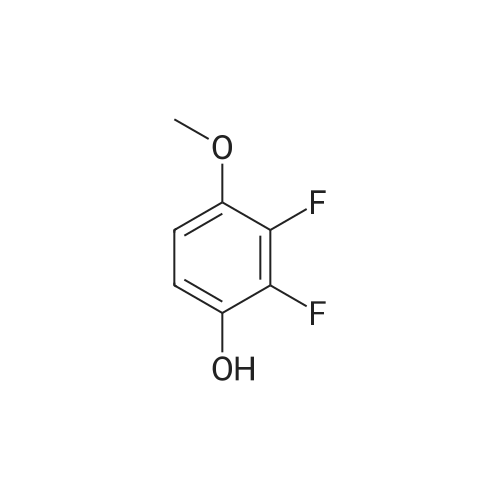
A solution of 31 .9 g (199 mmol) 2,3-dif luoro-4-methoxyphenol in 450 mL of dimethylsulfoxide was treated with 7.96 g (199 mmol) of sodium hydride and stirred at room temperature for 1 hour. Then 16 g (33.2 mmol) of 4-{6-bromo- 8-[(3,3,3-trifluoropropyl)amino]imidazo[1 ,2-b]pyridazin-3-yl}-N-cyclopropyl-2- methylbenzamide, which was prepared according to intermediate example 31a, was added and the mixture was heated overnight at 130C. After cooling, 300 mL ethyl acetate were added and the organic phase is washed with water. After evaporation of the organic phase, the residue was triturated with 200 mL EtOH to give 12.05 g (65%) of the title compound. 1 H-NMR (DMS0-d6): delta= 0.47-0.53 (2H), 0.62-0.70 (2H), 2.11 (3H), 2.72 (2H), 2.80 (1 H), 3.64 (2H), 3.92 (3H), 6.22 (1 H), 7.12 (1 H), 7.18 (1 H), 7.27 (1 H), 7.63 (1 H), 7.72 (1 H), 7.75-7.81 (1 H), 7.97 (1 H), 8.24 (1 H) ppm. |
| 65% |
|
A solution of 31.9 g (199 mmol) 2,3-difluoro-4-methoxyphenol in 450 mL of dimethylsulfoxide was treated with 7.96 g (199 mmol) of sodium hydride and stirred at room temperature for 1 hour. Then 16 g (33.2 mmol) of 4-{6-bromo-8- [(3,3,3-trifluoropropyl)amino]imidazo[1 ,2-b]pyridazin-3-yl}-N-cyclopropyl-2- methylbenzamide, which was prepared according to intermediate example 2a, was added and the mixture was heated overnight at 130C. After cooling, 300 mL ethyl acetate were added and the organic phase is washed with water. After evaporation of the organic phase, the residue was triturated with 200 mL EtOH to give 12.05 g (65%) of the title compound. 1 H-NMR (DMSO-d6): delta= 0.47-0.53 (2H), 0.62-0.70 (2H), 2.11 (3H), 2.72 (2H), 2.80 (1 H), 3.64 (2H), 3.92 (3H), 6.22 (1 H), 7.12 (1 H), 7.18 (1 H), 7.27 (1 H), 7.63 (1 H), 7.72 (1 H), 7.75-7.81 (1 H), 7.97 (1 H), 8.24 (1 H) ppm. |
| 65% |
|
Compound A27 N^yclopropy l-4-{6-(2, 3 iif luoro-4-methoxyphenoxy )-8-[ (3, 3, 3- trifluoro ropyl)amino]imidazo[1 ,2-b]pyridazin-3-yl}-2-methylbenzami A solution of 31.9 g (199 mmol) 2,3-difluoro-4-methoxyphenol in 450 mL of dimethylsulfoxide was treated with 7.96 g (199 mmol) of sodium hydride and stirred at room temperature for 1 hour. Then 16 g (33.2 mmol) of 4-{6-bromo- 8-[(3,3,3-trifluoropropyl)amino]imidazo[1 ,2-b]pyridazin-3-yl}-N-cyclopropyl-2- methylbenzamide, which was prepared according to intermediate example 27a, was added and the mixture was heated overnight at 130C. After cooling, 300 mL ethyl acetate were added and the organic phase is washed with water. After evaporation of the organic phase, the residue was triturated with 200 mL ethanol to give 12.05 g (65%) of the title compound. 1 H-NMR (DMSO-d6): delta= 0.47-0.53 (2H), 0.62-0.70 (2H), 2.11 (3H), 2.72 (2H), 2.80 (1 H), 3.64 (2H), 3.92 (3H), 6.22 (1 H), 7.12 (1 H), 7.18 (1 H), 7.27 (1 H), 7.63 (1 H), 7.72 (1 H), 7.75-7.81 (1 H), 7.97 (1 H), 8.24 (1 H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping