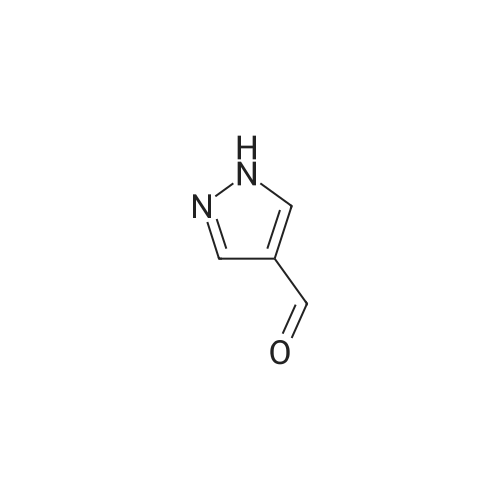
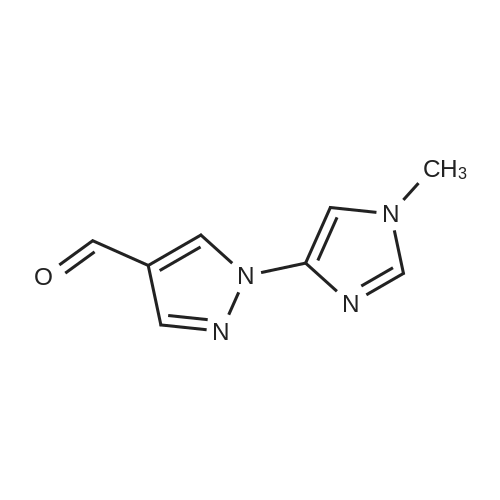
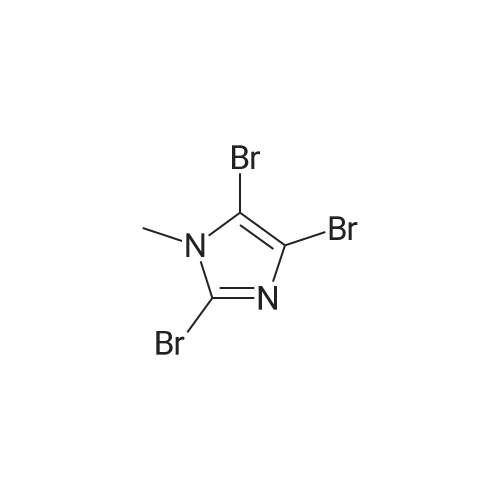
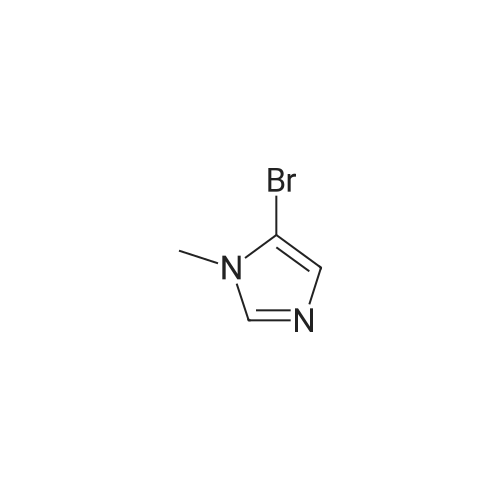
| 571 mg |
With sodium sulfite; for 24h;Reflux; |
To a solution of N-methylimidazole (1.64 g, 19.97 mmol) and sodium acetate (25 g, 300 mmol) in acetic acid (180 mL) at room temperature was added bromine (9.6 g, 60.07 mmol) dropwise as a solution in 20 mL acetic acid. The resulting mixture was stirred for 2.5 h at room temperature. Acetic acid was removed in vacuo, the residue was suspended in 500 mL water and stirred at room temperature for 10 minutes. The resultant precipitate was filtered, washed with water and dried under high vacuum to give 2,4,5-tribromo-l-methyl- lH-imidazole (1.82 g, 29percent - some product remained in the mother liquor) as a light yellow powder. Used without further characterization. To a suspension of the tribromide (1.82 g, 5.71 mmol) in 45 mL water was added sodium sulfite (13 g, 103 mmol) and the resulting mixture was stirred at rapid reflux for 24 h. After cooling to room temperature, organics were extracted with ether (3 chi 75 mL), dried over magnesium sulfate, filtered and concentrated to give 1.61 g of a mixture of tri-, di- and monobromoimidazoles. This mixture was re-subjected to the reduction conditions (same quantity of sodium sulfite) using 15 mL of 3: 1 water/acetic acid as solvent and heating in a sealed vessel at 130 °C for 60 h. After cooling to room temperature, the pH of the reaction mixture was adjusted to 9-10 by addition of 2 N sodium hydroxide. Organics were extracted with ether (3 chi 50 mL), dried over magnesium sulfate, filtered and concentrated to give crude 4-bromo-l -methyl- lH-imidazole (571 mg, ca. 62percent). Used without further characterization.. 4-Butyl-l -methyl- lH-imidazole (95 mg, 22 percent) was synthesized as in Example 3.1 using 4-bromo-l -methyl- lH-imidazole (571 mg, ca. 3.53 mmol) in place of 5-bromo-2- formylfuran and propylboronic acid (372 mg, 4.24 mmol) in place of hexylboronic acid. Used without further characterization. To a solution of diisopropylamine (0.13 mL, 0.918 mmol) in 2 mL anhydrous tetrahydrofuran at - 0°C was added -butyllithium (0.34 mL, 2.5 M in hexanes) dropwise. The solution was stirred while warming to -20 °C over 20 minutes. After cooling to -78 °C, 4-butyl-l -methyl- lH-imidazole (95 mg, 0.765 mmol) was added dropwise as a solution in 2 mL anhydrous tetrahydrofuran. The resulting solution was stirred for 40 minutes at -78 °C. Dimethylformamide (0.24 mL, 3.06 mmol) was added and the solution stirred while warming to room temperature. The reaction mixture was poured into 15 mL of 1 N hydrochloric acid and stirred for 5 minutes. The pH of the reaction mixture was adjusted to 7-8 by careful addition of saturated sodium bicarbonate solution. Organics were extracted with dichloromethane (3 chi 20 mL), dried over magnesium sulfate, filtered and concentrated. The crude residue was subjected to chromatography on silica gel with gradient elution (5-50percent ethyl acetate in hexanes) to give l -methyl-4-propyl-lH-imidazole-2-carbaldehyde (9 mg, 8percent) as an off-white solid. Used without further characterization. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping