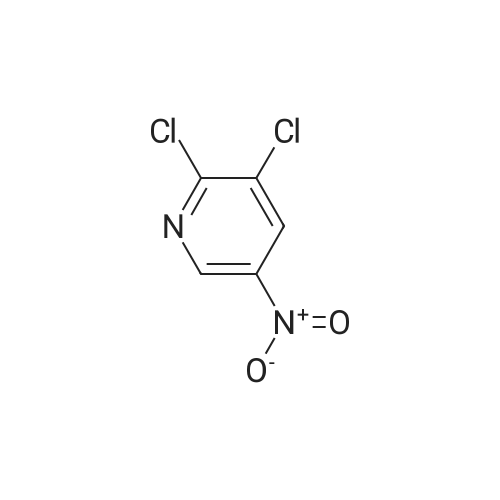
| 94.4% |
With trichlorophosphate; at 80℃; for 3h; |
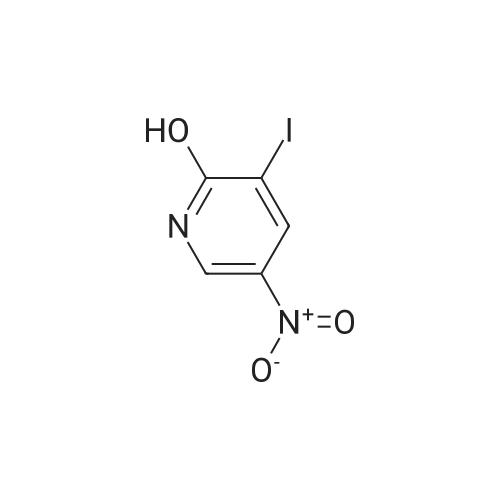
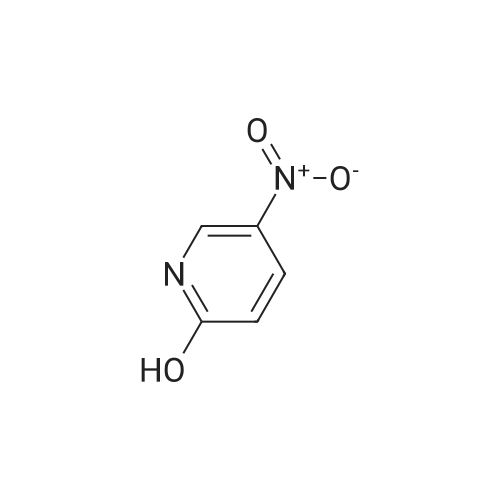
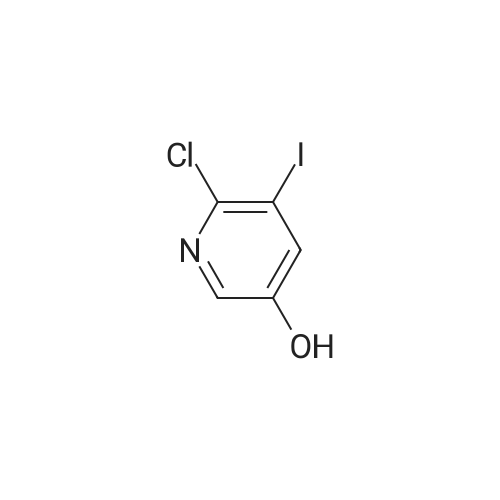
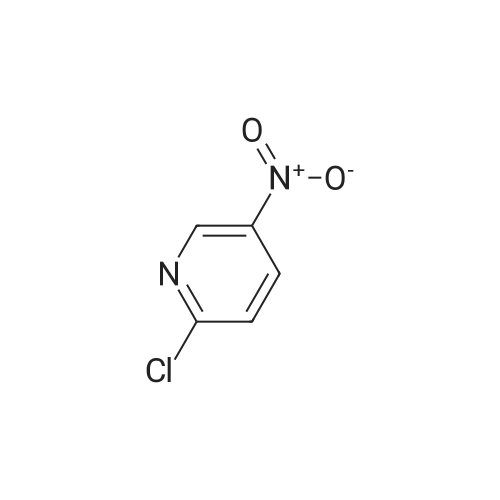
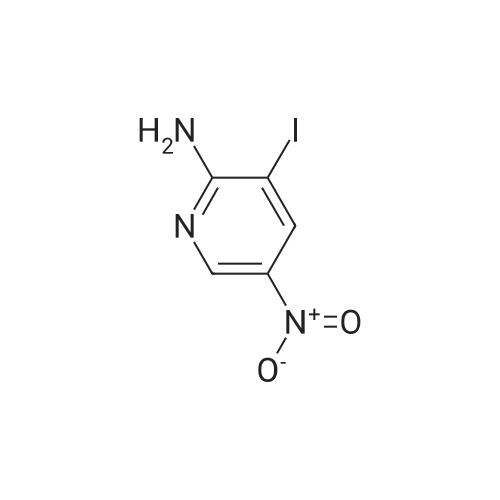
2-Hydroxy-3-iodo-5-nitropyridine (1.0 g, 3.76mmol) and phosphorus oxychloride (3.5 g, 11.28mmol) were charge, and the temperature was raised to 80C. The reaction was carried out at 80C for 3 hours and the mixture was poured into ice water. The precipitated crystals were filtered to give the title compound (1.01 g, 94.4%). |
| 81% |
With trichlorophosphate; In quinoline; at 120℃; for 2h; |
Synthesis of 2-Chloro-3-iodo-5-nitro-pyridine (CXXII): To a solution of CXXI (14 g, 52.6 mmol) in quinoline (5 mL) was added phosphorous oxychloride (4.8 mL, 52.2 mmol). The resulting mixture was heated at 120 C for 2 h. Progress of the reaction was monitored by 15 TLC. After cooling to room temperature the reaction mixture was poured onto ice cold water (75 mL) and the precipitate that formed was collected by filtration. The filtered solid was washed with water and allowed to dry to obtain CXXII (12 g, 81%) as a brown solid.1H NMR (400 MHz, CDCl3): delta 9.12 (d, J= 2.3 Hz, 1H), 8.84 (d, J= 2.4 Hz, 1H). |
| 69% |
With phosphorus pentachloride; trichlorophosphate; at 140℃; for 0.75h;Product distribution / selectivity; |
Intermediate 1; 6-Phenyl-5-(trifluoromethyl)pyridin-3-amineA. 2-Chloro-3-iodo-5-nitropyridineA mixture of <strong>[25391-58-6]3-iodo-5-nitropyridin-2-ol</strong> (37.60 mmol, 10 g), POCI3 (86.47 mmol, 7.94 ml) and PCI5 (48.87 mmol, 10.2 g) was heated at 14O0C for 45 minutes under argon atmosphere. The mixture was cooled at room temperature, poured slowly over iced-water and extracted with dichloromethane. The organic phase was washed with water, NaHCO3 aqueous solution and brine. The solvent was evaporated and the crude mixture was purified by chromatography over SiO2 eluting hexane/DCM mixtures affording 7.32 g (yield 69%) of the expected product. <n="39"/>1H NMR (300 MHz, CDCI3) delta ppm: 8.90 (s, 1 H), 9.19 (s, 1H). Intermediate 4A. 2-Chloro-3-iodo-5-nitropyridineA mixture of <strong>[25391-58-6]3-iodo-5-nitropyridin-2-ol</strong> (37.6 mmol, 10 g), POCI3 (86.47 mmol, 7.94 ml) and PCI5 (48.87 mmol, 10.2 g) was heated at 14O0C for 1h, under argon atmosphere. The crude mixture was poured into a mixture of ice and water and extracted with DCM. The solid residue was purified by chromatography over SiO2 eluting with hexane/dichloromethane mixtures affording 2-chloro-3-iodo-5-nitropyridine (7.32 g, yield69%) of the expected product. 1H NMR (300 MHz, CDCI3) delta ppm: 8.91 (d, J=2.47 Hz, 1H) 9.19 (d, J=2.47 Hz, 1H). Intermediate 606-Chloro-5-(trifluoromethyl)pyridin-3-amineA. 2-Chloro-3-iodo-5-nitropyridine A mixture of <strong>[25391-58-6]3-iodo-5-nitropyridin-2-ol</strong> (37.60 mmol, 10 g), POCI3 (86.47 mmol, 7.94 ml) and PCI5 (48.87 mmol, 10.2 g) was heated at 14O0C for 45 minutes under argon atmosphere. The mixture was cooled at room temperature, poured slowly over iced-water and extracted with dichloromethane. The organic phase was washed with water, NaHCO3 aqueous solution and brine. The solvent was evaporated and the crude mixture was purified by chromatography over SiO2 eluting hexane/DCM mixtures affording 7.32 g (yield 69%) of the expected product. delta 1H NMR (300 MHz, CDCI3): 8.90 (s, 1H), 9.19 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +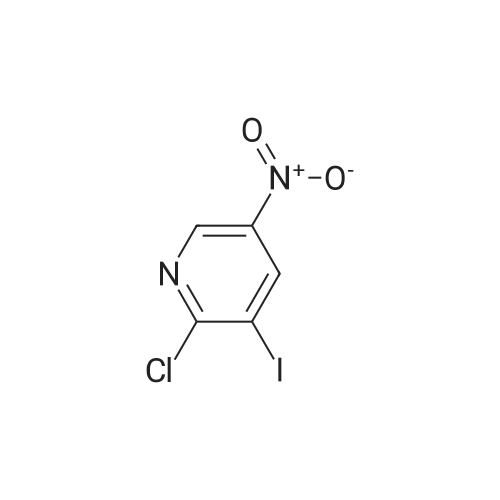

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping