| 93% |
With methanesulfonic acid; In i-Amyl alcohol; at 130 - 135℃; for 18h; |
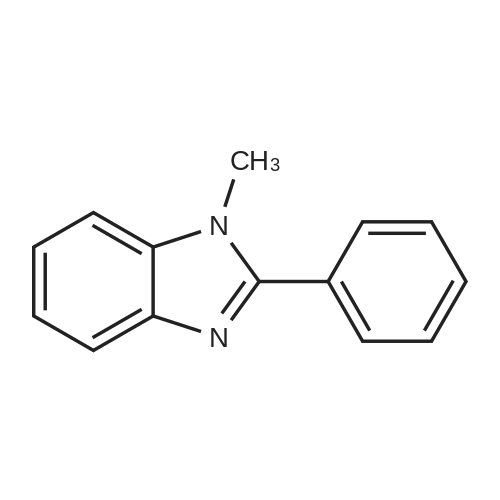
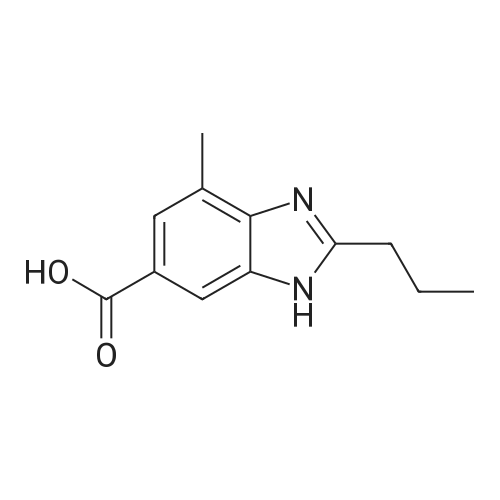
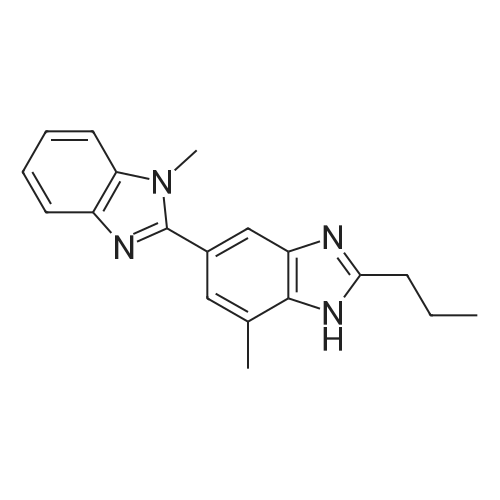
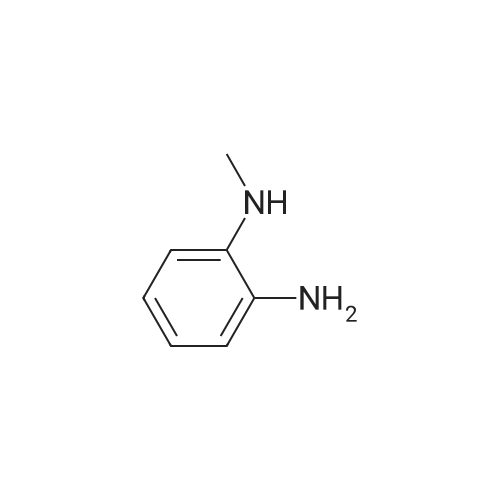
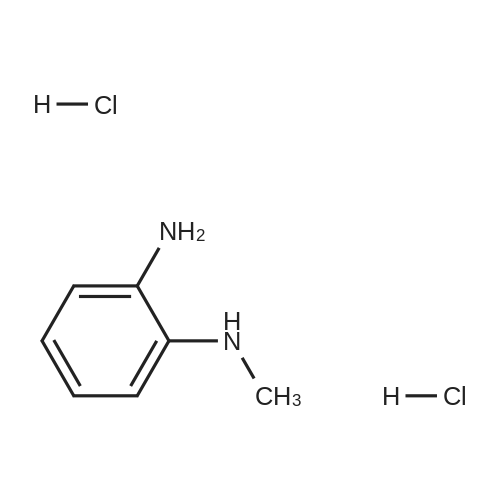
1.2 kg of isoamyl alcohol was added to the reaction flask.Add <strong>[152628-03-0]2-n-propyl-4-methyl-6-carboxybenzimidazole</strong> (400 g, 1.83 mol) and stir.Methanesulfonic acid (176 g, 1.83 mol, 1.0 eq) was added and N-methyl-phenylenediamine hydrochloride (360 g, 1.85 mol) was added.The temperature was raised to 130-135 C to reflux and the reaction was carried out for at least 18 hours until no significant moisture was separated. After the reaction is completed, cool to about 70 C,Add 2000 ml of water, stir, adjust the pH to 6~7 with 30% NaOH solution, stir and cool to 20~25 C after completion, and filter to obtain the product, the yield is 93.0%, and the HPLC purity is 99.6%. |
| 77.4% |
|
4-Methyl-2-n-propyl-lH-benzimidazole-6-carboxylic acid (50 gms) is suspended in Poly phosphoric acid (300 gms), temperature is raised and maintained for 30 min at 70 - 750C, N-Methyl-o-phenylenediamine dihydrochloride. (45 gms) is added lot wise over 2 hrs and maintained at temperature of 70 - 750C for lhr. The temperature of the reaction mass is raised and maintained for 10 hrs at 130 - 1350C. Mass temperature is cooled to 7O0C, water (600 ml) is added slowly at temperature of 60 - 9O0C. Temperature of the reaction mass is cooled to 3O0C, pH is adjusted to 8.0 - 8.5 with aqueous ammonia solution. EPO <DP n="12"/>Temperature of the reaction mass is raised, maintained at 50- 550C for 1 hr, filter the solid, wet cake is washed with hot water (200 ml) and unload the wet cake. The above wet cake suspended in water (900 ml), temperature is raised and mixed for 1 hr at 50 - 550C. Filtered the solid, washed with hot water (100 ml) and dried the wet cake at temperature of 70 - 750C till constant weight. The above dry material is suspended in methanol (260 ml), and temperature is raised to 45 - 5O0C, charcoal (6.5 gms) is added and mixed for about 30 min. Insolubles are filtered through hyflow bed, washed the bed with hot methanol (60 ml), collect and cooled the filtrate to 250C. Water (160 ml) is added slowly to the filtrate at temperature of 25 - 350C, Mass temperature is raised, maintained for 1 hr at reflux temperature. Reaction mass temperature is cooled, maintained for 2 hrs at 0 - 50C. The solid obtained is filtered, wet cake is washed with methanol (60 ml), the wet cake is dried at temperature of 70 - 750C till becomes constant weight. The dry weight of 4-Methyl-6(l -methyl benzimidazol-2-yl)-2-n-propyl IH- benzimidazole is 54 gms (Yield 77.4%). Water content by KF is 5.85%. |
|
With polyphosphoric acid; at 150℃; for 14h; |
General procedure: A solution of an appropriate ester (25.01mmol) in methanol (25mL) was added to a solution of NaOH (2.0g) in water (25mL), and the mixture was heated under reflux for 2h. After evaporation of methanol, the pH was adjusted to 4-5 by addition of aqueous citric acid. The precipitated solid was filtered, washed with ethanol and dried to yield carboxylic acid. The resulting compound was dissolved in polyphosphoric acid (10mL) at 150C. N-Methyl-o-phenylenediamine dihydrochloride (3.65g, 18.8mmol) was added to the mixture for 4 times in 4h. After stirring at 150C for 10h, the mixture was cooled and then poured into ice water (30mL). The pH was adjusted to 10 by addition of concentrated ammonia (ice cooling). The precipitated solid was filtered off, dried, and boiled in ethyl acetate (300mL). After cooling, the precipitated solid was filtered off, washed with diethyl ether, and dried to give the product as white solid.4.1.2.3 2-Propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)benzimidazole (8c) [14] 8c was prepared by following the above general procedure. Yield: 60.1%. MP: 193-195 C. 1H NMR (400 MHz, CDCl3) delta: 7.82 (d, 2H), 7.30-7.53 (m, 4H), 3.95 (s, 3H), 2.89 (t, 2H), 2.61 (s, 3H), 1.79 (m, 2H), 0.91 (t, 3H). MS (ESI): [M + H]+ calcd 305.2; found 305.1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping