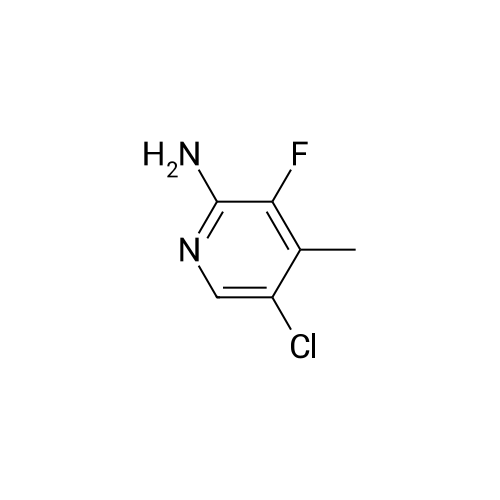
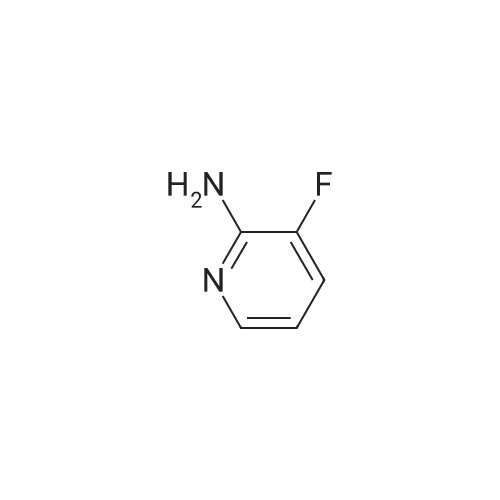
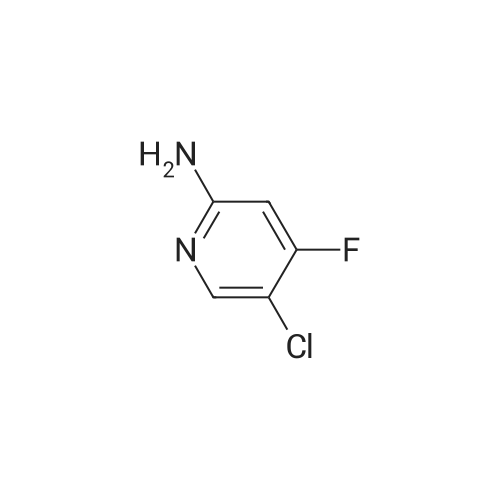
| 71% |
|
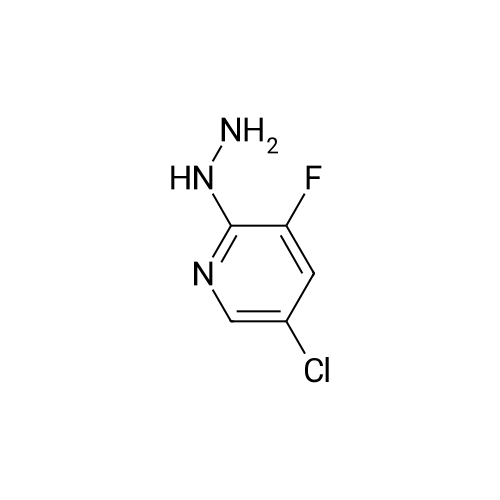
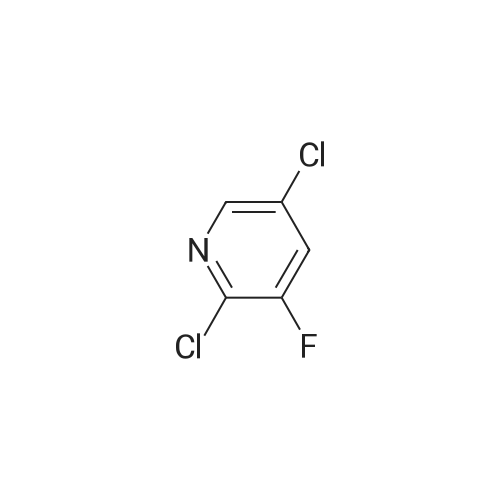
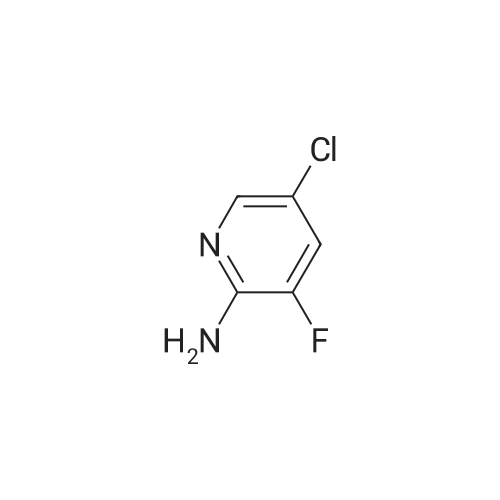
<strong>[246847-98-3]5-chloro-3-fluoro-pyridine-2-amine</strong> (147.0 mg, 1.0 mmol) was added to tetrahydrofuran (5 mL),Nitrogen was replaced three times, and the temperature was reduced to -78 C with a dry ice-acetone bath, and n-butyllithium (1.3 mL, 2.5 mmol, 2M) was slowly added dropwise to the reaction mixture, while controlling the reaction temperature to not exceed -55 C.After the addition was complete, the reaction mixture was stirred at -78 C for 1.5 hours.To the reaction mixture was added a solution of iodine (1.3 g, 5.1 mmol) in tetrahydrofuran (5 mL) (control the reaction temperature not to exceed -55 C). After the addition was complete, the reaction mixture was stirred at -78 C for 30 minutes.The reaction mixture was quenched by the dropwise addition of a saturated sodium thiosulfate solution,Stir for 10 minutes, allow to stand, separate the layers, extract the aqueous phase with ethyl acetate, combine the organic phases and wash twice with saturated sodium thiosulfate, dry, filter, and concentrate. The residue is purified by silica gel column chromatography (elution Agent: ethyl acetate / petroleum ether = 1/3),The title compound (194.0 mg, yield: 71%) was obtained in this step. |
| 70.4% |
|
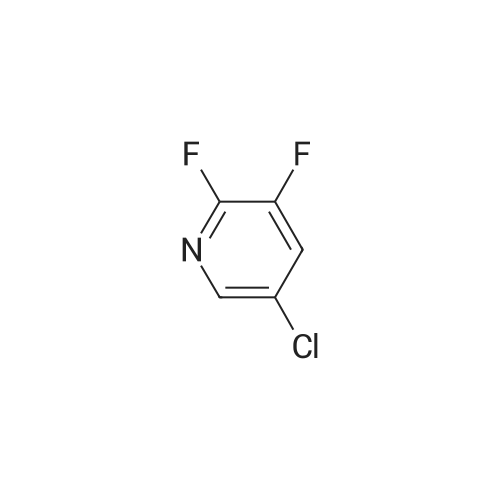
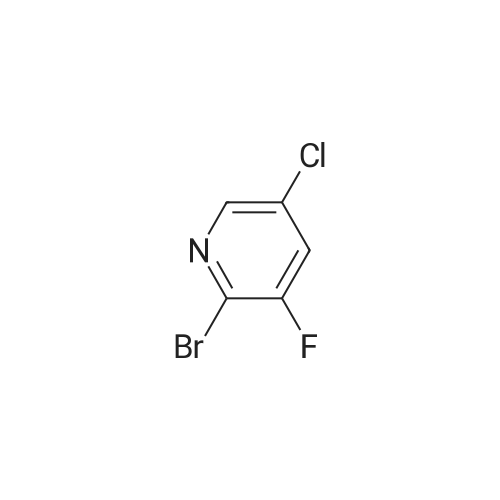
Compound 1-1 (1.0 g, 6.82 mmol, 1.00 eq) was dissolved in THF (40 mL). The solution was cooled with a dry ice/acetone bath. (The inner temperature was cooled to -65C). To this mixture was added n-BuLi (2.5 M, 6.82 mL, 2.50 eq) over 25 minutes while the inner temperature was controlled below -55C. After stirring the reaction for 90 minutes at -78 C, a solution of I2 (7.79 g, 30.69 mmol, 6.18 mL, 4.50 eq) in THF (20 mL) was added to the reaction quickly. The reaction was stirred for 15 minutes, then quenched with concentrated aqueous sodium thiosulfate pentahydrate (150 mL) and stirred for 10 minutes. Then the mixture was poured into ethyl acetate (200 mL) and the organic layer was separated. The aqueous layer was extracted with ethyl acetate (200 mL × 2) and the combined organic layer was dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 5:1 to 3:1) to afford compound 1-2 (1.44 g, 70.4% yield) as a brown solid. LC/MS (M + H+) m/z: calcd.272.90, found 272.9.1H NMR (400 MHz, CDCl3, delta): 7.86 (s, 1H), 4.67 (bs, 2H). |
| 45% |
|
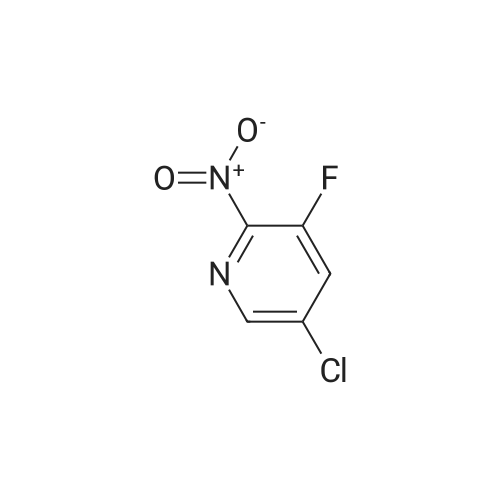
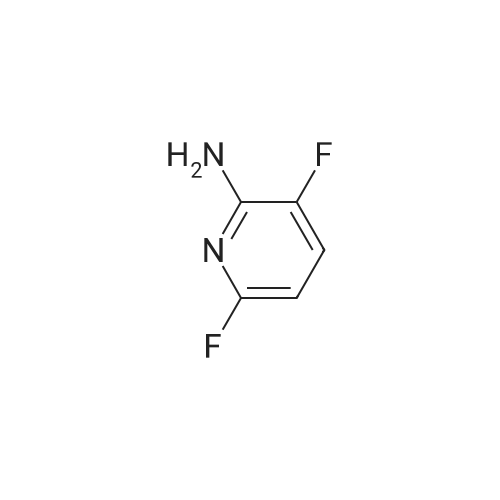
Example 5(b) 5-Chloro-3-fluoro-4-iodopyridin-2-amine. 5-Chloro-3-fluoropyridin-2-amine (11.7 g, 80 mmol, 1 equiv) was dissolved in THF (400 mL), cooled to -78 C., and stirred for 5 minutes. To this mixture was added n-BuLi (100 mL, 200 mmol, 2.5 equiv, 2.0 M in pentanes) over 10 minutes. After stirring the reaction for 90 minutes at -78 C., iodine (90.7 g, 360 mmol, 4.5 equiv dissolved in 200 mL of THF and cooled to ca -20 C.) was quickly added to the reaction. The reaction was stirred for 10 minutes and then quenched with concentrated aqueous sodium thiosulfate pentahydrate (ca 1.5 L). The mixture was poured into ethyl acetate (2 L). The organic layer was separated, and the aqueous layer was washed with ethyl acetate (400 mL). The combined organic layers were then dried over Na2SO4, filtered, and concentrated. The residue was dissolved in CH2Cl2 and purified by silica gel chromatography (50-90% CH2Cl2/hexanes) to yield 11.7 g of reddish solid. Trituration of the resultant solid with 10% CH2Cl2/hexanes afforded the title compound (10.0 g, 45%) as an off-white solid. m/z=272 [M+H]+ |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping