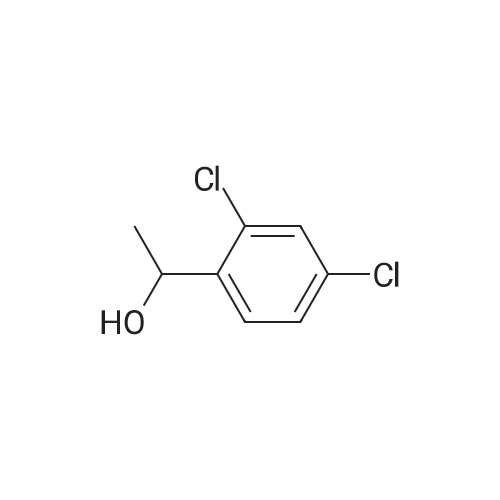
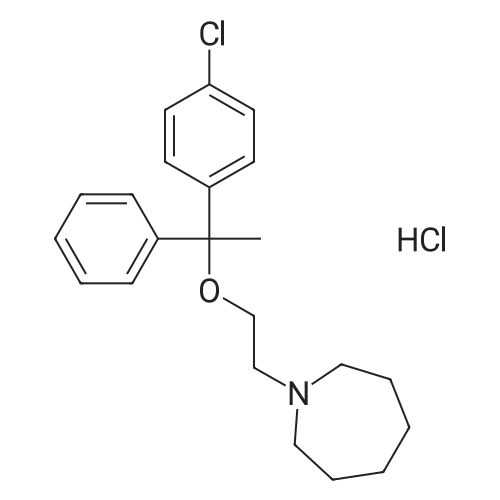
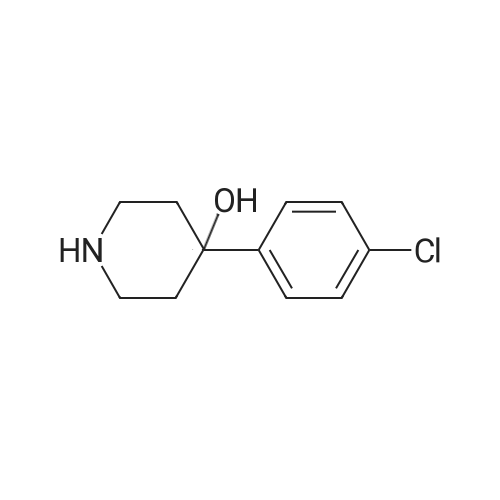
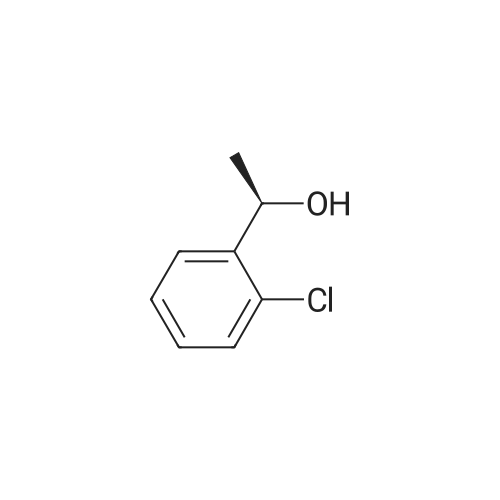
| 85.1% |
|
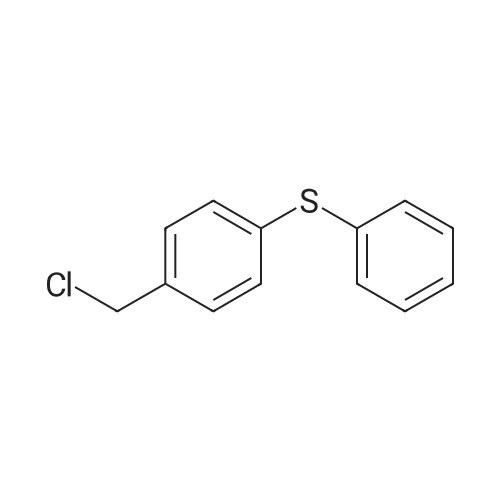
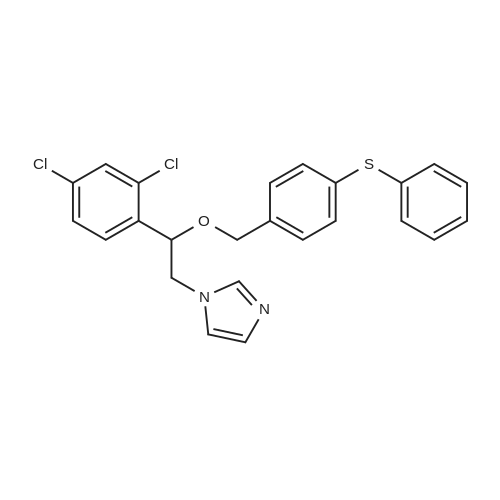
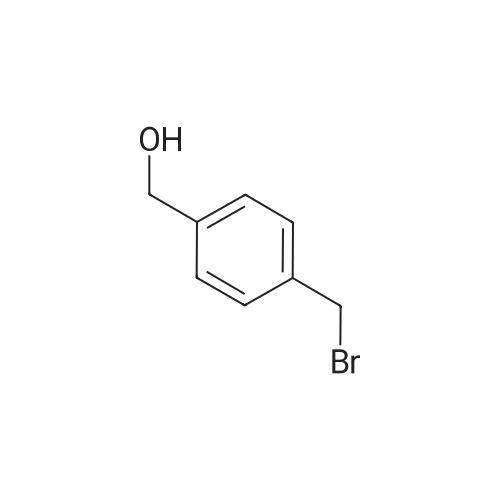
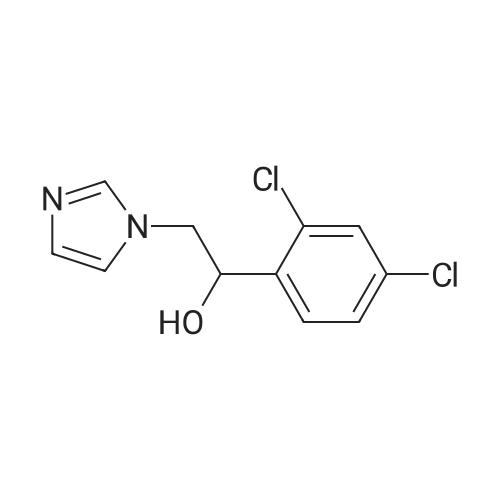
710 g of sodium hydroxide, 511.2 g of 1- (2,4-dichlorophenyl) -2-imidazole ethanol, 1136 ml of water, 5680 ml of toluene and 56.8 g of tetrabutylammonium bromide were placed in a room temperature (25 ° C.) 10L reaction flask, stirring was warmed to 58 ° C, a solution of <strong>[1208-87-3]4-phenylmercaptobenzyl chloride</strong> in toluene (554.1g ? 1130ml) was added dropwise over 1.5 hours. Reaction at 60 ° C 12hour,The layers were separated and the aqueous layer was discarded. The organic layer was washed with saturated aqueous sodium chloride solution three times (250 ml × 1 + 200 ml × 2), washed twice with water (200 ml × 2), dried over anhydrous magnesium sulfate, filtered and the filtrate was reduced in pressure , Temperature 55 ° C) was concentrated to dryness and toluene was recovered to obtain 1250.5 g of an oil. 5680 ml of a mixed solution (ethyl acetate: toluene = 1: 1) was added followed by 1136 ml of a 20percent nitric acid ethyl acetate solution and stirred at 25 ° C 3 hours, filtered, washed, the filter cake was collected, dried under atmospheric pressure at 40 ° C to give a light yellow solid 738g. Yield 71.4percent, HPLC pureDegree: 99.84percent.Into a 5000 ml reaction flask, 2800 ml of anhydrous ethanol, 340 ml of water, and 738 g of crude nitetazole were injected, and the temperature was raised to 60° C. to completely dissolve the solution. The solution was naturally cooled to 25° C., crystallized for 12 hours, filtered, and the filter cake was washed with a little ethyl acetate. , Drying under atmospheric pressure at 45°C gave 628 g of a white solid. Yield: 85.1percent, purity HPLC: 99.97percent. |
| 82.1% |
|
In 1000ml of three necked flask equipped with a stirrer, thermometer, dropping funnel added 25.6g of 1-(2,4-dichlorophenyl)-2-imidazol-ethanol (0.1mol) , 120ml tetrahydrofuran, 40ml water, 6g (0. 15mol) of sodium hydroxide ,1.2g PEG-400 and slowly warmed to reflux. While stirring added drop wise 4-Phenylthio-benzylchloride 30g (0. 12 mol 95percent), completion of the addition in approximately 30min and stirring at constant temperature for 4h,then the reaction was cooled, added 400ml of Diethyl ether, separatedaqueous layer, then aqueous layer was extracted with diethyl ether (200ml X2), combined the organic layer, washed with water , dried over anhydrous sodiumsulfate, filtrate , at the room temperature in filtrate liquid slowy & dropwise added 10ml concentrated nitric acid, precipitate solid, suction filtered,washed with diethyl ether, recrystallized using 95percent of ethanol to obtain white solid 37.3g, yield 82.1percent. mp 136-138oC. |
| 74.7% |
|
At room temperature (25 °C) Sodium hydroxide, 1- (2,4-dichlorophenyl) -2-imidazol ethanol, water, toluene, tetrabutylammonium bromide administered into the reaction flask, heated with stirring to 58 °C 4-phenyl benzyl benzyl chloride in toluene was added dropwise, and the addition was completed in 1.5 h. Insulation reaction 12h, layers were separated aqueous layer was discarded, and the organic layer was washed with a saturated aqueous sodium chloride solution, washed with water, the toluene layer was dried over anhydrous magnesium sulfate, filtered and the filtrate under reduced pressure (pressure of 1330 Pa, a temperature of 55 °C) and concentrated to dryness recovered toluene to give an oil, was added a mixed solvent (ethyl acetate: toluene = 1: 1), added 20percent nitric acid in ethyl acetate solution at room temperature (25 °C) was stirred for 3h crystallization, filtration, water washing, collecting The filter cake was dried under normal pressure at 40 °C to give a pale yellow solid |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping