| 83% |
|
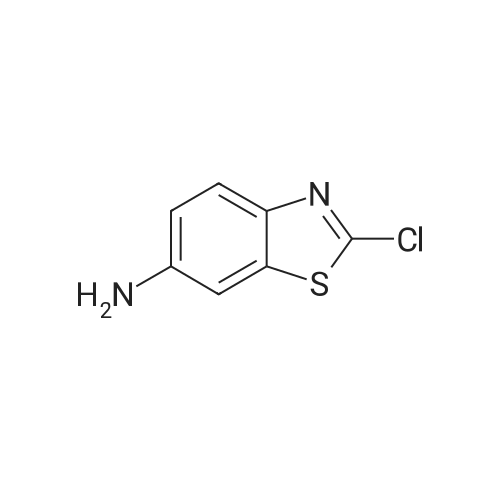
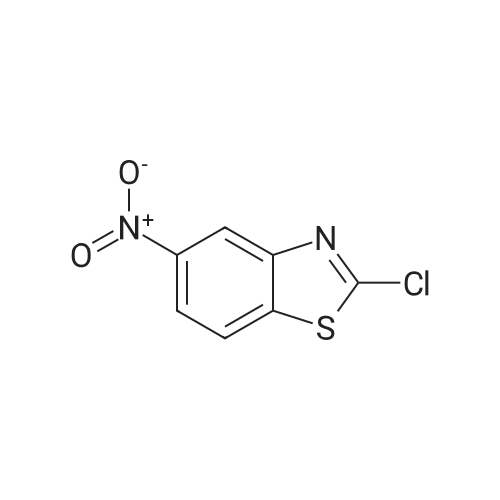
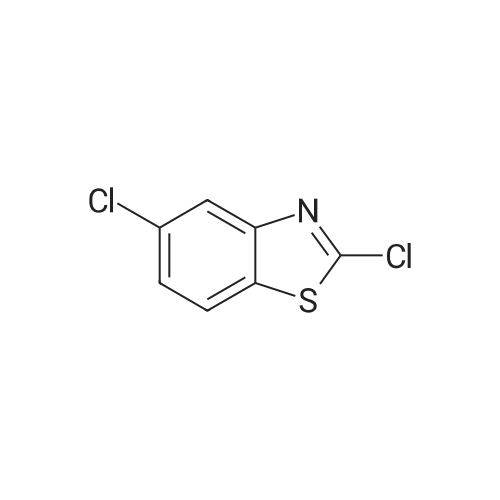
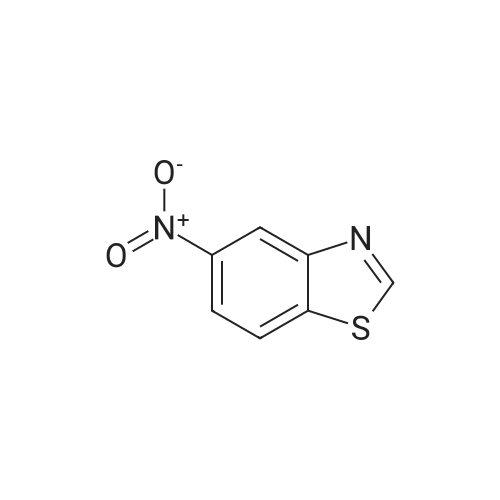
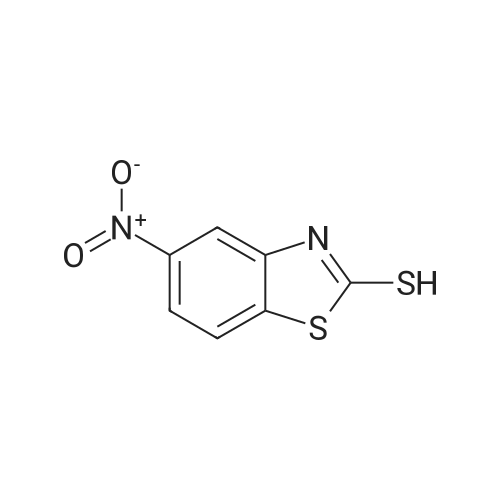
To a solution of 2-CHLOROBENZOTHIAZOLE (12.0 g, 70.7 MMOL) in concentrated H2SO4 (60 mL) was added HN03 (69% solution, 6 mL) dropwise at 0C for 20 min. The mixture was stirred at 5C for 3h, poured into ice-water (150 mL). The precipitate was collected and washed with 5% sodium bicarbonate and water, dried in VACUO.'H NMR analysis showed the mixture contained 78% 6-nitro-2-chlorobenzothiazole and 8% 5-nitro-2- chlorobenzothiazole. Recrystallization from ethanol gave 6-nitro-2-chlorobenzothiazole as white crystalline solid (11 g, 72%). 3.5 g of the solid was dissolved in refluxing ethanol-acetic acid (150: 15 mL), Iron powder was added in one portion.. The mixture was refluxed for 1.5h, filtered. The filtrate was concentrated in vacuo to half volume and neutralized with 10% NaOH to pH 7.5, extracted with ethyl acetate. The organic phase was washed with brine, dried over magnesium sulphate and evaporated to give a residue, which was RECRYSTALLIZED from ethanol. Light purple crystals (2.5 g, 83%) were obtained. Mp 160-164C ; TLC single spot at Rf 0.27 (30% EtOAc/hexane) ;'HNMR (270 MHz, DMSO-d6) 5 7.58 (1H, d, J = 9.0 Hz, 4-H), 7.03 (1H, d, J = 2.0 Hz, 7-H), 6.77 (1 H, dd, J = 9.0, 2. 0 Hz, 5-H), 5.55 (2H, s, NH2). The mother liquor from the RECRYSTALLIZATION of nitration product was evaporated and subjected to iron powder reduction as described above. The crude product was purified with flash chromatography (ethyl acetate-DCM gradient elution) to give 2-CHLORO- benzothiazol-5-yl-amine as yellow solid. Mp 146-149C ; TLC single spot at Rf 0.52 (10% EtOAc/DCM) ;'HNMR (270 MHZ, DMSO-d6) 8 7.63 (1 H, d, J = 8. 6 HZ, 7-H), 7.05 (1 H, d, J = 2.3 Hz, 4-H), 6.78 (1 H, dd, J = 8.6, 2.3 Hz, 6-H), 5.40 (2H, s, NH2). |
| 61% |
|
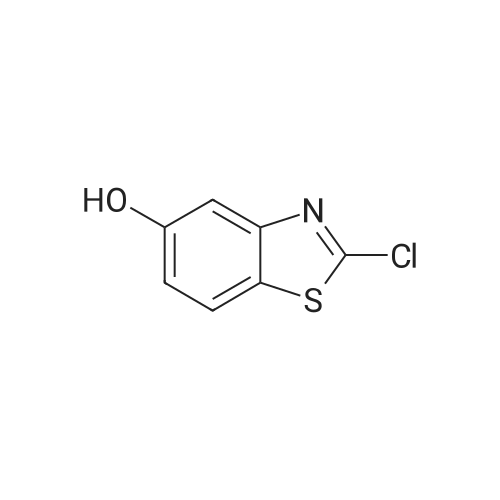
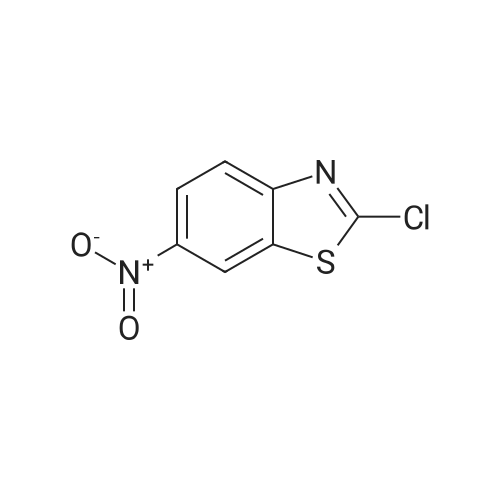
(B) Synthesis of 2-chloro-6-aminobenzothiazole (3) Compound 2 (1.96 g, 9.14 mmol) was dissolved in ethanol (150 mL) and purified water (100 mL), and the solution was added with anhydrous tin(II) chloride (20.7 g, 91.7 mmol). The mixture was added with 4.8 mol/L hydrochloric acid (20 mL, 96 mmol) and refluxed at 120 C. After disappearance of the starting materials was confirmed by thin layer chromatography (developing solvent: dichloromethane), the mixture was basified with aqueous sodium hydroxide. The precipitates were removed by filtration, After, ethanol was evaporated, the residue was extracted three times with ethyl acetate. Then organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (developing solvent: ethyl acetate/n-hexane=1/1) to obtain Compound 3 as white solid (1.02 g, 61% yield). 1H-NMR (300 MHz, CDCl3) delta 3.85 (br, 2H), 6.81 (dd, 1H, J=2.4, 8.7 Hz), 6.99 (d, 1H, J=2.4 Hz), 7.70 (d, 1H, J=8.7 Hz). MS (ESI+) 185.0, [M+H]+. |
| 33% |
With iron; acetic acid; at 40℃; for 5h; |
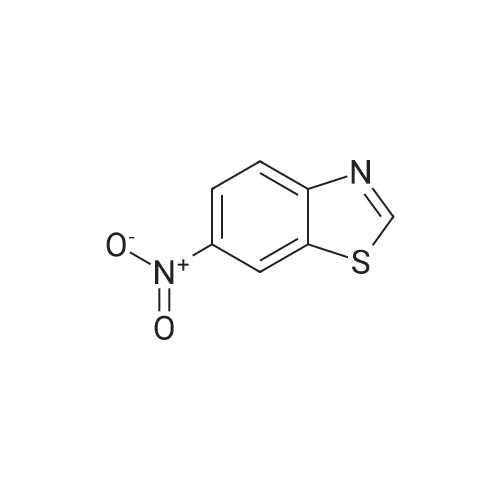
Suspend 2-Chloro-6-nitro-benzothiazole (21.43 g, 99.8 mmol) in glacial acetic acid (30OmL). Add elemental iron (12.9 g, 231 mmol) and stir at 40 0C for 5 h. Filter the reaction mixture through Celite, concentrate in vacuo, and adsorb onto silica gel. Subject the residue to silica gel flash column chromatography in two portions [(120 g column, 0-10% CH3OH/CH2CI2), (120 g column, 0-5% CH3OHZCH2Cl2)] to yield the desired product (6.17 g, 33%). mass spectrum (m/e): 185.0 (M+l). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping