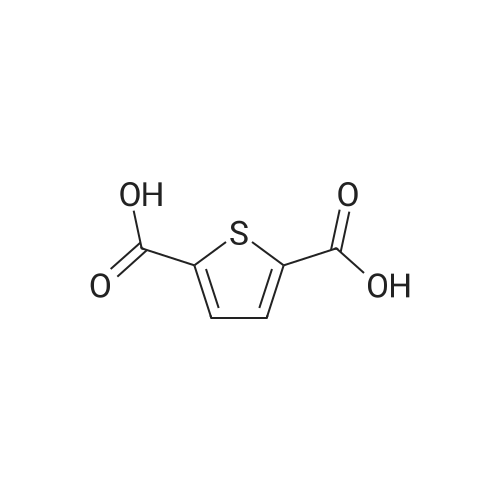
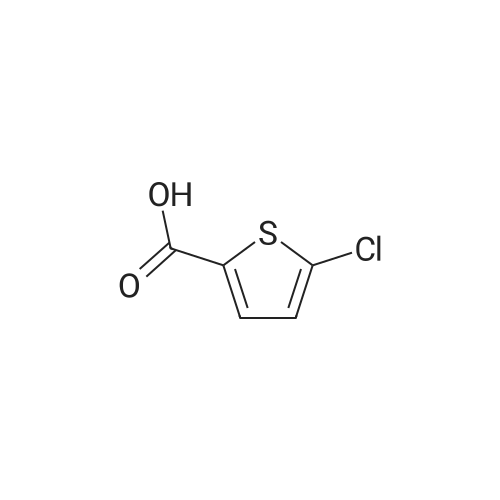
| 91% |
With sulfuric acid; for 6h;Heating / reflux; |
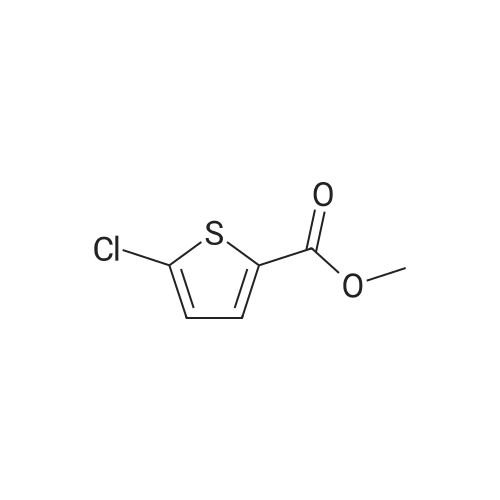
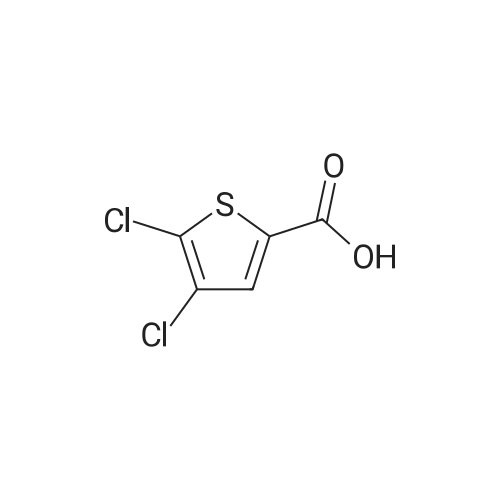
Step 1: Production of methyl 5-chlorothiophene-2-carboxylate To a solution of 5-chlorothiophene-2-carboxylic acid (5.07 g, 31.2 mmol) in methanol (30 ml) was added conc. sulfuric acid (2 ml), and the mixture was heated under reflux for 6 hr. After allowing to cool to room temperature, water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed successively with water, saturated aqueous sodium hydrogen carbonate solution, and saturated brine, and dried over anhydrous magnesium sulfate. After filtration, the solvent was evaporated under reduced pressure to give methyl 5-chlorothiophene-2-carboxylate (4.99 g, yield 91%) as a crude product. 1H-NMR(400MHz, deltappm, DMSO-d6): 7.58(1H, d, J=8.0Hz), 6.93(1H, d, J=4.0Hz), 3.87(3H, s). |
| 73% |
With sulfuric acid;Reflux; |
General procedure: 5-Halide thiophene-2-carboxylate (1equiv) was solved in 3ml absolute methanol, and concentrated sulfuric acid (2.2equiv) was added. The reaction mixture was heated under reflux conditions overnight. After cooling to room temperature, the solvent was evaporated. Purified water was added, and the reaction mixture was neutralized with saturated NaHCO3. After extraction with dichloromethane, the combined organic phase was washed with purified water, 5% NaHCO3 and brine. The organic layer was dried over MgSO4 and filtrated. The solvent was removed under reduced pressure, and the white solid phase was used without any further purification. |
| 53.3% |
With sulfuric acid; for 28h;Reflux; |
3.716 g (22.9 mmol) TEPS 62 were solved in 30 mL MeOH and 1.5 ml_ cone. H2S04 were added. After 28 hours of stirrig with reflux cooling, NaOH was added to alkalize the solution. Afterwards, the solution was extracted with ethyl acetate three times and dried over Na2S04. Then the solvent was removed on a rotary evaporator, yielding a liquid brown product of 2.157 g (53.3 %). 1H NMR (200 MHz, chloroform-of) d 7.59 (d, J = 4.0 Hz, 1 H) d 6.93 (d, J = 4.0 Hz, 1 H) d 3.87 (s, 3H). 13C NMR (50 MHz, DMSO-d6) d 160.8, 135.6, 133.8, 131.3, 128.6, 52.5. MS m/z: 176 M+ |
|
sulfuric acid; at 64℃; for 16h;Industry scale; Inert atmosphere; |
Example 3; Preparation of Compound G The 200-gallon reactor was charged with methanol (135 kg), 5-chlorothiophene-2-carboxylic acid F (12 kg, 73.81 mol, 1.0 equiv), and sulfuric acid (6.7 kg, 68.31 mol) under nitrogen. The contents were warmed to reflux (64 C.) for 16 hours and reaction completion monitored by HPLC. The reactor was cooled to 40 C. and the mixture was vacuum distilled to an oil at <50 C. The methyl ester obtained was cooled to 20 C. and ammonium hydroxide (157 kg, 2586 mol, 35.2 equiv) was charged along with heptane (10 kg) to the reactor. The reactor contents were mixed for 36 hours at ambient temperature. The completion of the reaction was monitored by HPLC for the disappearance of methyl ester intermediate. The precipitated solids were centrifuged and washed with water followed by heptane. The isolated solids were dried at 45 C. for 14 hours to afford Compound G (8.42 kg, 77.1% yield). |
|
With sulfuric acid; at 64℃; for 16h;Industry scale; Inert atmosphere; |
Example 3 Preparation of Compound III-B The 200-gallon reactor was charged with methanol (135 kg, 14.3 volumes), 5-chlorothiophene-2-carboxylic acid III-G (12 kg, 73.81 mol, 1.0 equiv), and sulfuric acid (6.7 kg, 68.31 mol) under nitrogen. The contents were warmed to reflux (64 C.) for 16 hours and reaction completion monitored by HPLC. The reactor was cooled to 40 C. and the mixture was vacuum distilled to an oil at <50 C. The methyl ester obtained was cooled to 20 C. and ammonium hydroxide (157 kg, 2586 mol, 35.2 equiv) was charged along with heptane (10 kg) to the reactor. The reactor contents were mixed for 36 hours at ambient temperature. The completion of the reaction was monitored by HPLC for the disappearance of methyl ester intermediate. The precipitated solids were centrifuged and washed with water followed by heptane. The isolated solids were dried at 45 C. for 14 hours to afford Compound III-B (8.42 kg, 77.1% yield). |
|
With sulfuric acid; at 64℃; for 16h;Industry scale; Inert atmosphere; |
Example 3: Preparation of Compound GH2S04 NH3 (28% aq) Q C ^,S C02H MeOH ClySyC02Me Heptane/r.t, 37hrs S JL64C for l 6 rJs 71.1 % yieldF G[0134] The 200-gallon reactor was charged with methanol (135 kg), 5-chlorothiophene- 2-carboxylic acid F (12 kg, 73.81 mol, 1.0 equiv), and sulfuric acid (6.7 kg, 68.31 mol) under nitrogen. The contents were warmed to reflux (64 C) for 16 hours and reaction completion monitored by HPLC. The reactor was cooled to 40 C and the mixture was vacuum distilled to an oil at < 50 C. The methyl ester obtained was cooled to 20 C and ammonium hydroxide (157 kg, 2586 mol, 35.2 equiv) was charged along with heptane (10 kg) to the reactor. The reactor contents were mixed for 36 hours at ambient temperature. The completion of the reaction was monitored by HPLC for thedisappearance of methyl ester intermediate. The precipitated solids were centrifuged and washed with water followed by heptane. The isolated solids were dried at 45C for 14 hours to afford Compound G (8.42 kg, 77.1% yield). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping