| 70% |
|
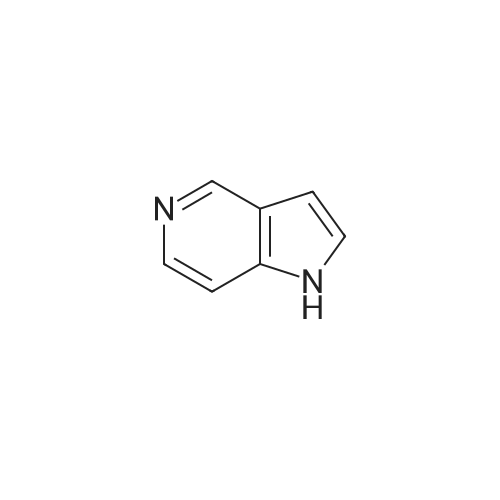
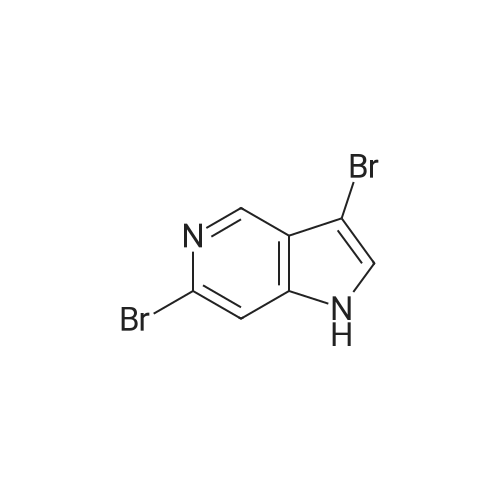
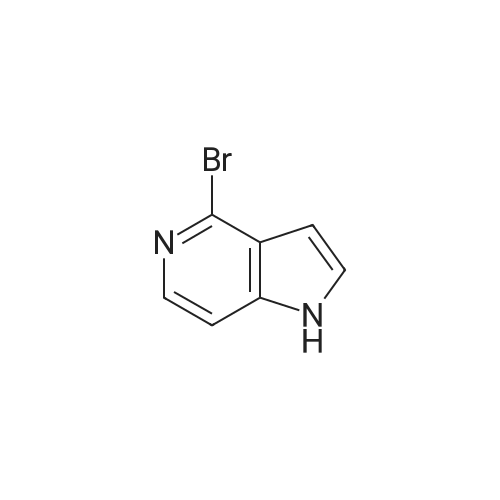
Example 1 3-bromo-<strong>[271-34-1]5-azaindole</strong>[00145] Referring now to the Scheme 1 as shown in Fig. 1 , a solution of 3.0128g(25.51 mmol) of <strong>[271-34-1]5-azaindole</strong> (Atlantic SciTech Group) in 50 ml. of acetonitrile was placed in a 250 ml. three-neck round-bottom flask equipped with a magnetic stirrer, thermocouple, nitrogen bleed, and cooling ice bath. A total of 17.1Og (76.53 mmol, 3 eg.) of solid CuBr2 was added portion-wise to the flask at 17C in 10 min. The resulting green suspension was stirred at room temperature until no starting material was observed by TLC (approximately 1-2 hours, Rf = 0.23 for <strong>[271-34-1]5-azaindole</strong> and 0.49 for 3- bromo-<strong>[271-34-1]5-azaindole</strong> in EtOAc/MeOH=9:1 ). The reaction mixture was cooled to 10C and then was slowly quenched by addition of IN ammonia in methanol solution (1 1OmL). The resulting blue solution was concentrated on rotavap at room temperature, and the residue was extracted with ethyl acetate (3 x 80 ml_). The organic extract was dried over Na2SO4, filtered, and concentrated to residual volume of about 50 ml_. The temperature of the residue was brought to reflux resulting in the dissolving of all solids, and then hexane was added to the hot mixture until crystallization occured. The resulting suspension was cooled on an ice bath, the product was filtered, washed with cold EtOAc/hexane = 1 :3 and then hexane, and dried. The 3-bromo-<strong>[271-34-1]5-azaindole</strong> was an off- white solid, yield 3.52 g (70% yield). |
| 51% |
With copper(II) bromide; In acetonitrile; at 0 - 20℃; |
To a solution of <strong>[271-34-1]5-azaindole</strong> (11.8 g, 0.1 mol) in MeCN (100 mL) was added cuprous bromide (67.0 g, 0.3 mol) at 0C. The green reaction mixture was warmed to room temperature, and stirred until the raw materials disappeared. The temperature of the reaction mixture was reduced to 0C with an ice bath, and a solution of ammonia in methanol (7N) was slowly added dropwise to the mixture to quench the reaction. The mixture was raised to room temperature and stirred for 0.5 hours, diluted with water and extracted with ethyl acetate (*3). The organic phases were combined and washed with saturated brine (*1), dried over anhydrous sodium sulfate, filtered, concentrated in vacuo, and purified by column chromatography to afford the title compound (10.0 g, yield 51%). 1HNMR (400 MHz, DMSO-d6) δ 11.90 (s, 1H), 8.78-8.33 (m, 2H), 7.69 (s, 1H), 7.47 (s, 1H). m/z=197[M+1]+. |
| 51% |
With copper(II) bromide; In acetonitrile; at 0 - 20℃; |
To a solution of <strong>[271-34-1]5-azaindole</strong> (11.8 g, 0.1 mol) in MeCN (100 mL) was added cuprous bromide (67.0 g, 0.3 mol) at 0C. The green reaction mixture was warmed to room temperature, and stirred until the raw materials disappeared. The temperature of the reaction mixture was reduced to 0C with an ice bath, and a solution of ammonia in methanol (7N) was slowly added dropwise to the mixture to quench the reaction. The mixture was raised to room temperature and stirred for 0.5 hours, diluted with water and extracted with ethyl acetate (*3). The organic phases were combined and washed with saturated brine (*1), dried over anhydrous sodium sulfate, filtered, concentrated in vacuo, and purified by column chromatography to afford the title compound (10.0 g, yield 51%). 1HNMR (400 MHz, DMSO-d6) δ 11.90 (s, 1H), 8.78-8.33 (m, 2H), 7.69 (s, 1H), 7.47 (s, 1H). m/z=197[M+1]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping