| 97% |
With phosphorus tribromide; In water; N,N-dimethyl-formamide; at 0℃; |
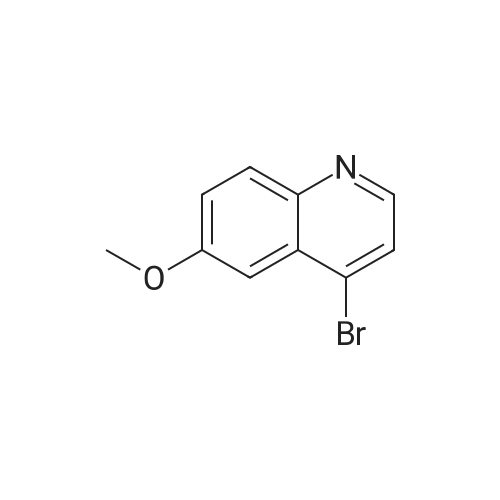
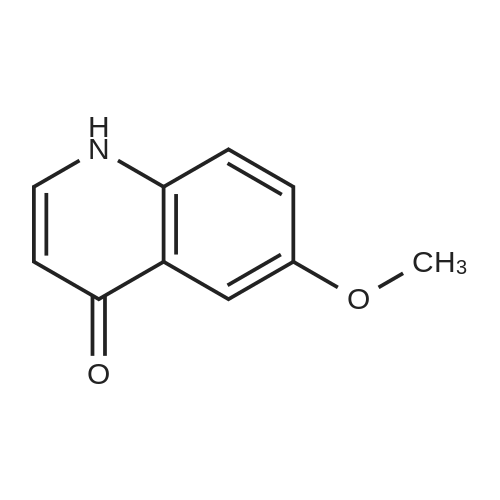
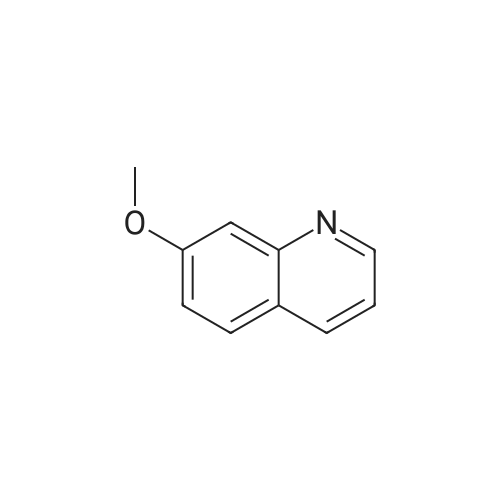
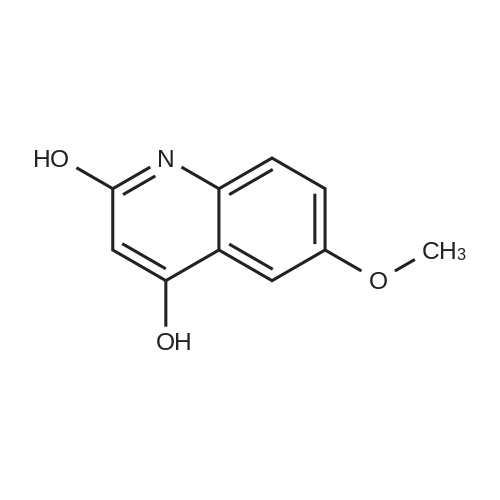
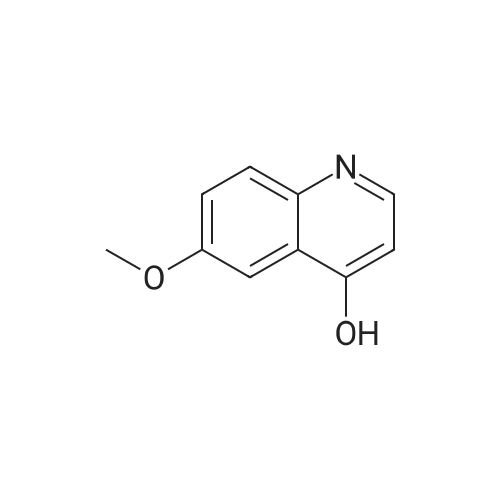
To a solution of 6-(methyloxy)-4-quinolinol (23.6 g, 135 mmol) in DNF (100 mL) was added PBr3 (16 mL, 169 mmol) in portions. The flask was filled with a bubbler. When bubbling ceased, the suspension was dumped into icy water with stirring. The resulting mixture was diluted with a large volume of water (-600 mL). The percipitate was filtered, washed with water and dried under vaccume line to afford the title compound as an off-white solid (31.4 g, 97%): LC/MS (ES) m/e 239 (M+H)+ |
| 95% |
With phosphorus tribromide; sodium hydroxide; In water; N,N-dimethyl-formamide; at 75℃; |
A three-necked round bottom flask vented to a 1M NaOH (aq) gas trap was charged with 4-hydroxy-6-methoxyquinoline (12.07 g, 68.90 mmol, 1.0 equiv) and anhydrous N,Ndimethylformamide(75 mL) and stirred magnetically. To the heterogeneous mixture was addedPBr3 (8.0 mL, 85 mmol, 1.2 equiv) drop/portionwise over several minutes by syringe. During theaddition, the internal temperature rose to 75 C, and gas was evolved. A copious precipitate formedtoward the end of the addition. The vent to the gas trap was removed 15 min after the completionof addition, and the reaction was stirred vigorously for an additional 1h 45 min, then quenched bypouring onto 150 g ice in 150 mL water. After brief stirring, Na2CO3 (20 g) was added in smallportions (bubbling), and the mixture was stirred for 15 min, whereupon the pH was approximately7. The taupe-colored product was isolated by vacuum filtration on a Buchner funnel, washingextensively with water. After drying for several days in a vacuum desiccator, the title compoundwas obtained in ca. 95% purity as a light tan, powdery solid (15.58 g, 65.44 mmol, 95%). |
| 95% |
With phosphorus tribromide; sodium hydroxide; In N,N-dimethyl-formamide; at 75℃; for 1.75h; |
A threenecked round bottom flask vented to a 1MNaOH (aq) gas trap was charged with 14 (12.07 g, 68.9() rnmol, 10 equiv) and anhydious NN-dimethy1forrnainide (75 mL)and stirred magnetically. To the heterogeneous mixture was added PBr3 (8.0 mL, 85 mmol,1.2 equiv) drop/porlionwise over several minutes by syringe. Duiing the addition, the internal temperature rose to 75 C, and gas was evolved. A copious precipitate formed toward the end of the addition. The vent to the gas trap was 15 mm after the completion of addition, and the reaction was stirred vigorously for an additional lh 45 mm, then quenchedby pouring onto 150 g ice in 150 mL water. Aer brief stirring, Na2CO3 (20 g) was added in small portions (bubbling), and the mixture was stirred for 15 mm, whereupon the pH was approximately 7. The taupecoiored product was isolated by vacuum filtration on a Buchner finnel. washing extensively with water. After drying for several days in a vacuum desiccator, the title compound was obtained in ca. 95% purity as a light tan, powdery solid (1558 g, 6544 mmol, 95%). ‘H NMR (300 MHz, DMSOd6) ?: 857 (d, J= 4.7, 1H); 8.00(d, J= 9.2, 111); 7.89 (d. ,J= 4.7, 1H); 7.51 (dd, J= 2.8, ). 2, IH); 7.39 (d, J= 2.8, 1H); 3.96 (S. 3H). (See W02006/0002047, which is incorporated by reference herein in its entirety.) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping