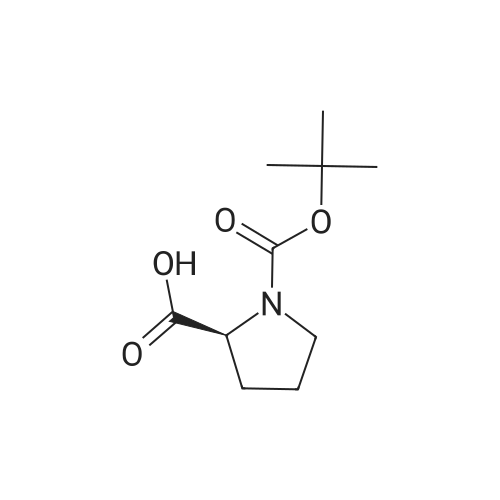
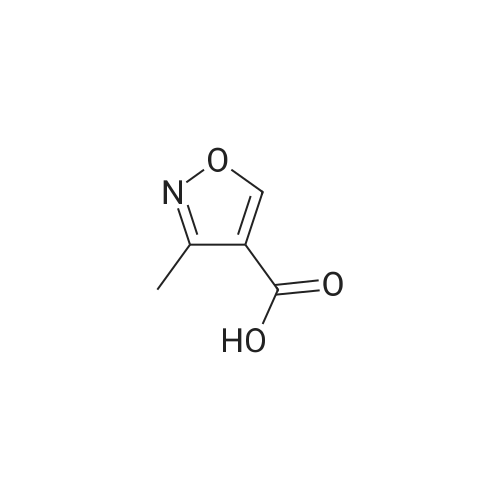
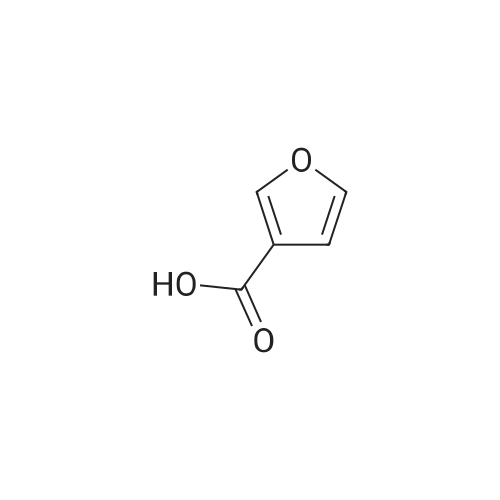
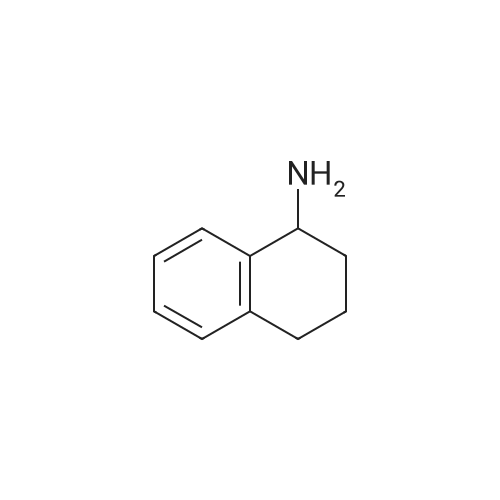
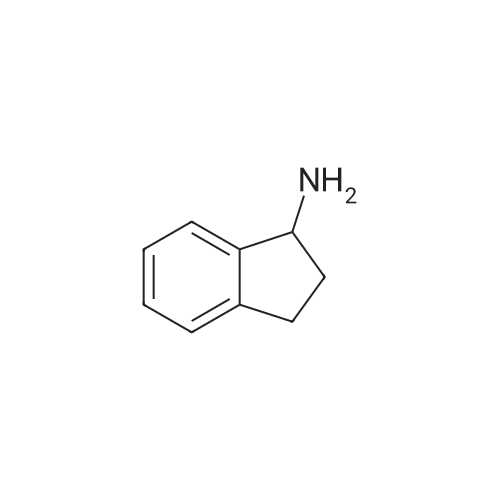
| 63% |
at 80℃; for 14h; |
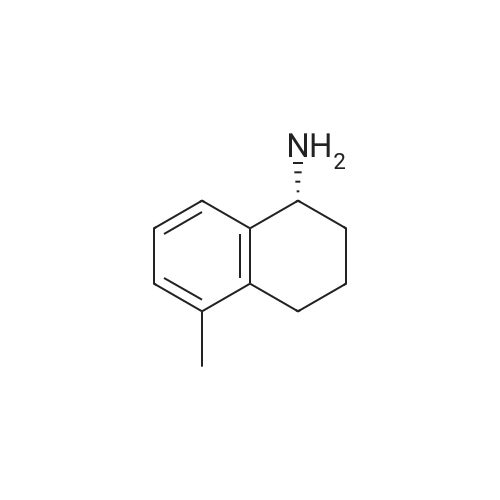
A mixture of(R)-(-)-1,2,3,4-tetrahydro-1-naphthylamine (10.0 g, 67.9 mmol, 1 equiv) and ethylformate (6.23 mL, 77.4 mmol, 1.14 equiv) was heated to 80 C for 14 h. Hexanes was added and themixture was triturated by sonication, then filtered and rinsed with hexanes to yield the product (7.44 g,63%) as atan solid. R1= 0.22 (3:1 hexanes/EtOAc). ‘HNMR(400 MHz, CDC13) 3: 8.23 (s, 1H), 7.29-7.25 (m, 1H), 7.23-7.16 (m, 2H), 7.13-7.08 (m, 1H), 5.82 (bs, 1H), 5.28 (dd, 1H, J= 5.2, 14.0 Hz), 2.85-2.73 (m, 2H), 2.15-2.03 (m, 1H), 1.88-1.81 (m, 3H); ‘3C NMR (100 MHz, CDC13) 3: 160.5, 137.7, 136.1,129.4, 128.8, 127.6, 126.5, 46.4, 30.3, 29.3, 20.0. HRMS calcd for C,,H,3NONa: 198.0889, found198.0890. |
|
at 60℃; |
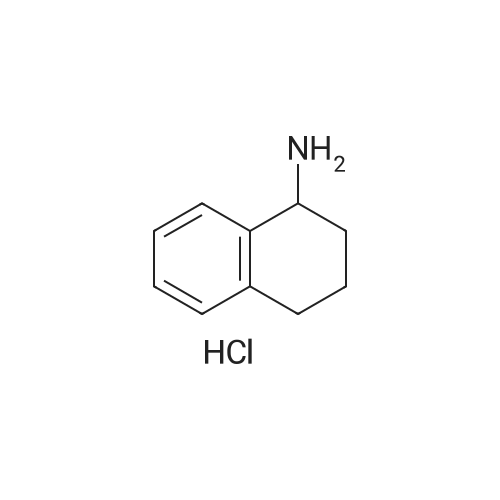
(l/?)-l,2,3,4-Tetrahydro-l-naphthalenamine (5.0 g, 34 mmol) was heated in 15 mL of ethyl formate at 60 C overnight, during which time a precipitate formed. The reaction mixture was added to 100 mL of hexanes with stirring, and the resulting solids were filtered, washed with hexanes and dried to give 4.63 g of the formamide as white needles. The resulting formamide (4.54 g, 26 mmol) was dissolved in 50 mL of tetrahydrofuran and added dropwise to a suspension of lithium aluminum hydride (1.1 g, 29 mmol) in 20 mL of tetrahydrofuran that had been cooled to 0 C. The reaction mixture was refluxed overnight, then cooled to 0 C and quenched by the sequential addition of 1.1 mL of water, 1.1 mL of15 % aqueous NaOH solution and 3.3 mL of water. The mixture was stirred at ambient temperature for 30 minutes and diluted with ethyl acetate. Several grams of MgSO4 were added, and the mixture was filtered through Celite diatomaceous filter aid and concentrated under reduced pressure to give 4.0 g of the title compound as a colorless oil.1H NMR (CDCl3) δ 1.17 (s, IH), 1.65-2.0 (m, 4H), 2.47 (s, 3H), 2.65-2.85 (m, 2H), 3.63 (m,IH), 7.0-7.35 (m, 4H). |
|
at 60℃; |
EXAMPLE 6 General preparation of 2-[1-[(substituted-phenyl)acetyl]-4-piperidinyl]-N-methyl-N-[(1R)-1,2,3,4-tetrahydro-1-naphthalenyl]-4-thiazolecarboxamide (Compound 144, Compound 145, Compound 146, Compound 147, Compound 148 and Compound 132) and N-methyl-2-[1-[[substituted-1H-pyrazol-1-yl]acetyl]-4-piperidinyl]-N-[(1R)-1,2,3,4-tetrahydro-1-naphthalenyl]-4-thiazolecarboxamide (Compound 149 and Compound 150) Step A: Preparation of (1R)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine. (1R)-1,2,3,4-tetrahydro-1-naphthalenamine (5.0 g, 34 mmol) was heated in 15 mL of ethyl formate at 60 0C overnight, during which time a precipitate formed. The reaction mixture was added to 100 mL of hexanes with stirring, and the resulting solids were filtered, washed with hexanes and dried to give 4.63 g of the formamide as white needles. The resulting formamide (4.54 g, 26 mmol) was dissolved in 50 mL of tetrahydrofuran and added dropwise to a suspension of lithium aluminum hydride (1.1 g, 29 mmol) in 20 mL of tetrahydrofuran that had been cooled to 0 C. The reaction mixture was refluxed overnight, then cooled to 0 0C and quenched by the sequential addition of 1.1 mL of water, 1.1 mL of 15 % aqueous NaOH solution and 3.3 mL of water. The mixture was stirred at ambient temperature for 30 minutes and diluted with ethyl acetate. Several grams of MgSO4 were added, and the mixture was filtered through Celite diatomaceous filter aid and concentrated under reduced pressure to give 4.0 g of the title compound as a colorless oil. 1HNMR (CDCl3) δ 1.17 (s, 1H), 1.65-2.0 (m, 4H), 2.47 (s, 3H), 2.65-2.85 (m, 2H), 3.63 (m, 1H), 7.0-7.35 (m, 4H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +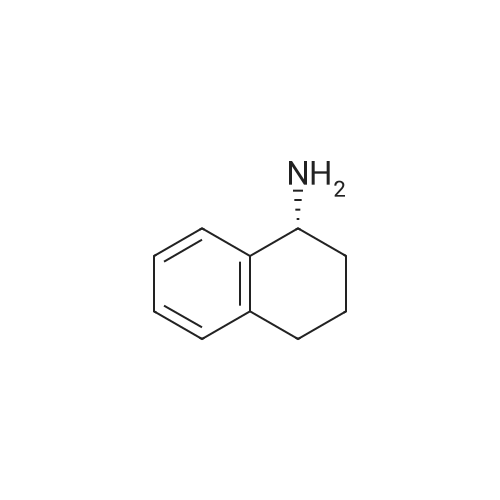

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


–H Primary Amination.png)