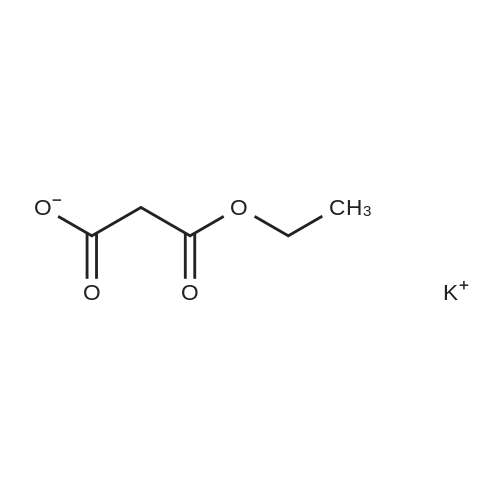
|
In ethanol; at 40 - 55℃; for 1 - 2.5h;Product distribution / selectivity; |
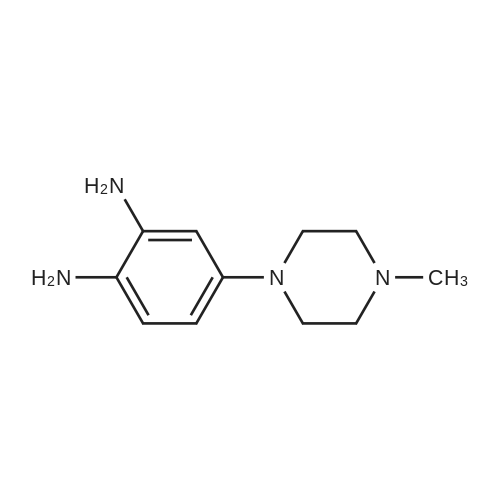
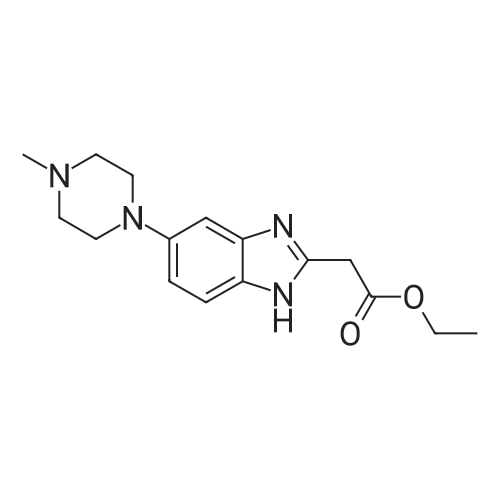
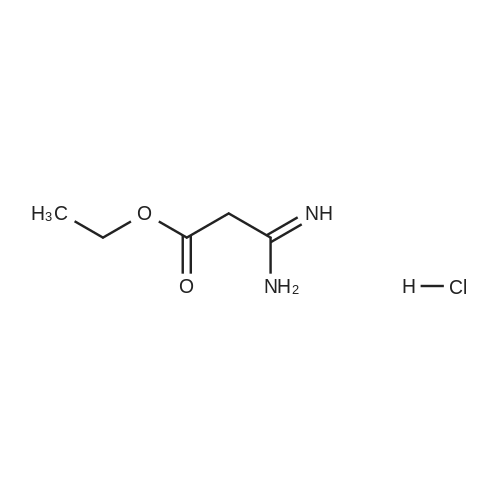
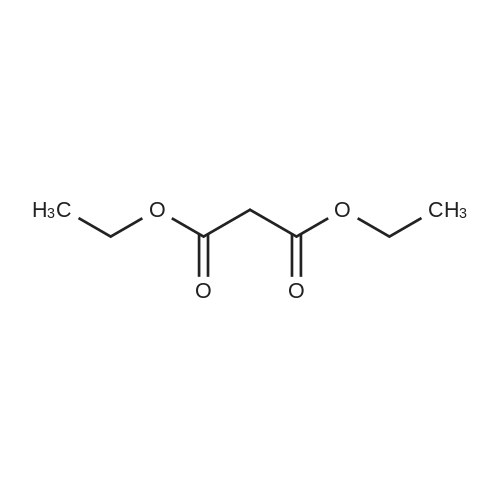
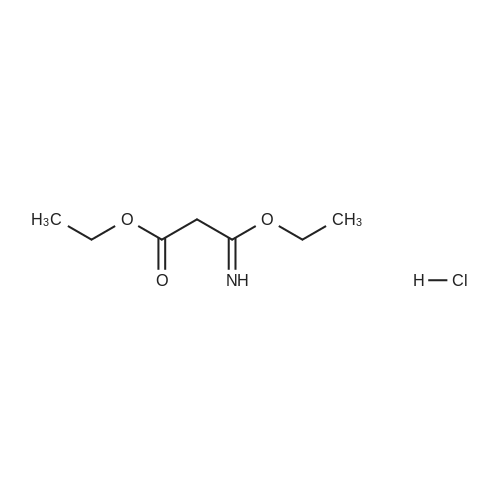
[0145] After all the 5-(4-methyl-piperazin-1-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. Next, 440.0 g (2.25 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added as a solid. The reaction was stirred at 40-50C (internal temperature) until the reaction was complete. The reaction was monitored by following the disappearance of the diamino compound by HPLC. The typical reaction time was 1-2 hours. After the reaction was complete, it was cooled to room temperature and filtered through a pad of Celite filtering material. The Celite filtering material was washed with absolute EtOH (2 x 250 mL), and the filtrate was concentrated under reduced pressure providing a thick brown/orange oil. The resulting oil was taken up in 850 , mL of a 0.37% HCI solution. Solid NaOH (25 g) was then added in one portion, and a precipitate formed. The resulting mixture was stirred for 1 hour and then filtered. The solid was washed with H2O (2 x 400 mL) and dried at 5O0C in a vacuum oven providing 251.7 g (74.1%) of [6-(4-methyl-piperazin-1-yl)-1 H- benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder.; [0147] After all the 5-(4-methyl-piperazin-1-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. The diamine intermediate is air sensitive so care was taken to avoid exposure to air. 500 g (2.56 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added to the reaction mixture over a period of about 30 minutes. The reaction was stirred at 45-55C . (internal temperature) under N2 until the diamine was completely consumed as determined by HPLC. The typical reaction time was about 2 hours. After the reaction was complete, the reaction was filtered while warm through a pad of Celite. The reaction flask and Celite were then washed with 200 proof EtOH (3 x 285 mL). The filtrates were combined in a 5000 mL flask, and about 3300 mL of ethanol was removed under vacuum producing an orange oil. Water (530 mL) and then 1 M HCL (350 mL) were added to the resulting oil, and the resulting mixture was stirred. The resulting solution was vigorously stirred while 30% NaOH (200 mL) was added over a period of about 20 minutes maintaining the internal temperature at about 25- 30C while the pH was brought to between 9 and 10. The resulting suspension was stirred for about 4 hours while maintaining the internal temperature at about 20-25C. The resulting mixture was filtered, and the filter cake was washed with H2O (3 x 300 mL). The collected solid was dried to a constant weight at 5O0C under vacuum in a vacuum oven providing 345.9 g (90.1%) of [6~(4-methyl- piperazin-1-yl)-1 H-benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder. In an alternative work up procedure, the filtrates were combined and the ethanol was removed under vacuum until at least about 90% had been removed. Water at a neutral pH was then added to the resulting oil, and the solution was cooled to about O0C. An aqueous 20% NaOH solution was then added slowly with rapid stirring to bring the pH up to 9.2 (read with pH meter). The resulting mixture was then filtered and dried as described above. The alternative work up procedure provided the light tan to light yellow product in yields as high as 97%. |
|
|
Procedure AA .5000 mL, 4-neck flask was fitted with a stirrer, thermometer, condenser, and gas inlet/outlet. The equipped flask was charged with 265.7 g (1.12 mol. 1.0 eq) of 5-(4-methyl- piperazin-l-yl)-2-nitroaniline and 2125 mL of 200 proof EtOH. The resulting solution was purged with N2 for 15 minutes. Next, 20.0 g of 5% Pd/C (50% H2O w/w) was added. The reaction was vigorously stirred at 40-500C (internal temperature) while H2 was bubbled through the mixture. The reaction was monitored hourly for the disappearance of 5-(4- methyl-piperazin-l-yl)-2-nitroaniline by HPLC. The typical reaction time was 6 hours.After all the 5-(4-methyl-piperazin-l-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. Next, 440.0 g (2.25 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added as a solid. The reaction was stirred at 40-500C (internal temperature) until the reaction was complete. The reaction was monitored by following the disappearance of the diamino compound by HPLC. The typical reaction time was 1-2 hours. After the reaction was complete, it was cooled to room temperature and filtered through a pad of Celite filtering material. The Celite filtering material was washed with absolute EtOH (2 x 250 mL), and the filtrate was concentrated under reduced pressure EPO <DP n="67"/>providing a thick brown/orange oil. The resulting oil was taken up in 850 niL of a 0.37% HCl solution. Solid NaOH (25 g) was then added in one portion, and a precipitate formed. The resulting mixture was stirred for 1 hour and then filtered. The solid was washed with H2O (2 x 400 mL) and dried at 500C in a vacuum oven providing 251.7 g (74.1%) of [6-(4- methyl-piperazin-l-yl)-lH-benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder.Procedure BA 5000 mL, 4-neck jacketed flask was fitted with a mechanical stirrer, condenser, temperature probe, gas inlet, and oil bubbler. The equipped flask was charged with 30O g (1.27 mol) of 5-(4-methyl-piperazin-l-yl)-2-nitroaniline and 2400 mL of 200 proof EtOH (the reaction may be and has been conducted with 95% ethanol and it is not necessary to use 200 proof ethanol for this reaction). The resulting solution was stirred and purged with N2 for 15 minutes. Next, 22.7 g of 5% Pd/C (50% H2O w/w) was added to the reaction flask. The reaction vessel was purged with N2 for 15 minutes. After purging with N2, the reaction vessel was purged with H2 by maintaining a slow, but constant flow of H2 through the flask. The reaction was stirred at 45-55C (internal temperature) while H2 was bubbled through the mixture until the 5-(4-methyl-piperazin-l-yl)-2-nitroaniline was completely consumed as determined by HPLC. The typical reaction time was 6 hours. After all the 5-(4-methyl-piperazin-l-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. The diamine intermediate is air sensitive so care was taken to avoid exposure to air. 500 g (2.56 mol) of ethyl 3-ethoxy-3- iminopropanoate hydrochloride was added to the reaction mixture over a period of about 30 minutes. The reaction was stirred at 45-55C (internal temperature) under N2 until the diamine was completely consumed as determined by HPLC. The typical reaction time was about 2 hours. After the reaction was complete, the reaction was filtered while warm through a pad of Celite. The reaction flask and Celite were then washed with 200 proof EtOH (3 x 285 mL). The filtrates were combined in a 5000 mL flask, and about 3300 mL of ethanol was removed under vacuum producing an orange oil. Water (530 mL) and then IM HCL (350 mL) were added to the resulting oil, and the resulting mixture was stirred. The resulting solution was vigorously stirred while 30% NaOH (200 mL) was added over a period of about 20 minutes maintaining the internal temperature at about 25-300C while the pH was brought to between 9 and 10. The resulting suspension was stirred for about 4 hours while maintaining the internal temperature at about 20-250C. The resulting mixture was filtered, EPO <DP n="68"/>and the filter cake was washed with H2O (3 x 300 mL). The collected solid was dried to a constant weight at 500C under vacuum in a vacuum oven providing 345.9 g (90.1%) of [6-(4- methyl-piperazin-l-yl)-lH-benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder. In an alternative work up procedure, the filtrates were combined and the ethanol was removed under vacuum until at least about 90% had been removed. Water at a neutral pH was then added to the resulting oil, and the solution was cooled to about O0C. An aqueous 20% NaOH solution was then added slowly with rapid stirring to bring the pH up to 9.2 (read with pH meter). The resulting mixture was then filtered and dried as described above. The alternative work up procedure provided the light tan to light yellow product in yields as high as 97%. |
|
at 40 - 55℃; for 0.25h;Product distribution / selectivity; |
Procedure A[0159] A 5000 imL, 4-neck flask was fitted with a stirrer, thermometer, condenser, and gas inlet/outlet. The equipped flask was charged with 265.7 g (1.12 mol. 1.0 eq) of 5-(4-methyl-piperazin-1-yl)-2-nitroaniline and 2125 mL of 200 proof EtOH. The resulting solution was purged with N2 for 15 minutes. Next, 20.0 g of 5% Pd/C (50% H2O w/w) was added. The reaction was vigorously stirred at 40-50C (internal temperature) while H2 was bubbled through the mixture. The reaction was monitored hourly for the EPO <DP n="57"/>disappearance of 5-(4-methyl-piperazin-1-yl)-2-nitroaniline by HPLC. The typical reaction time was 6 hours.[0160] After all the 5-(4-methyl-piperazin-1 -yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. Next, 440.0 g (2.25 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added as a solid. The reaction was stirred at 40-50C (internal temperature) until the reaction was complete. The reaction was monitored by following the disappearance of the diamino compound by HPLC. The typical reaction time was 1-2 hours. After the reaction was complete, it was cooled to room temperature and filtered through a pad of Celite filtering material. The Celite filtering material was washed with absolute EtOH (2 x 250 mL), and the filtrate was concentrated under reduced pressure providing a thick brown/orange oil. The resulting oil was taken up in 850 mL of a 0.37% HCI solution. Solid NaOH (25 g) was then added in one portion, and a precipitate formed. The" resulting mixture was stirred foτi hour and then filtered. The solid was washed with H2O (2 x 400 mL) and dried at 50C in a vacuum oven providing 251.7 g (74.1%) of [6-(4-methyl-piperazin-1-yl)-1 H- benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder.; Procedure B[0161] A 5000 mL, 4-neck jacketed flask was fitted with a mechanical stirrer, condenser, temperature probe, gas inlet, and oil bubbler. The equipped flask was charged with 300 g (1.27 mol) of 5-(4-methyl-piperazin-1- yl)-2-nitroaniline and 2400 mL of 200 proof EtOH (the reaction may be and has been conducted with 95% ethanol and it is not necessary to use 200 proof ethanol for this reaction). The resulting solution was stirred and purged with N2 for 15 minutes. Next, 22.7 g of 5% Pd/C (50% H2O w/w) was added to the reaction flask. The reaction vessel was purged with N2 for 15 minutes. After purging with N2, the reaction vessel was purged with H2 by maintaining a slow, but constant flow of H2 through the flask. The reaction was stirred at EPO <DP n="58"/>45-55C (internal temperature) while H2 was bubbled through the mixture until the 5-(4-methyl-piperazin-1-yl)-2-nitroaniline was completely consumed as determined by HPLC. The typical reaction time was 6 hours.[0162] After all the 5-(4-methyl-piperazin-1-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. The diamine intermediate is air sensitive so care was taken to avoid exposure to air. 500 g (2.56 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added to the reaction mixture over a period of about 30 minutes. The reaction was stirred at 45-55C (internal temperature) under N2 until the diamine was completely consumed as determined by HPLC. The typical reaction time was about 2 hours. After the reaction was complete, the reaction was filtered while warm through a pad of Celite. The reaction flask and Celite were then washed with 200 proof EtOH (3 x 285 mL). The filtrates were combined in a 5000 mL flask, and about 3300 mL of ethanol was removed under vacuum producing an orange oil. Water (530 mL) and then 1 M HCL (350 mL) were added to the resulting oil, and the resulting mixture was stirred. The resulting solution was vigorously stirred while 30% NaOH (200 mL) was added over a period of about 20 minutes maintaining the internal temperature at about 25-30C while the pH was brought to between 9 and 10. The resulting suspension was stirred for about 4 hours while maintaining the internal temperature at about 20-250C. The resulting mixture was filtered, and the filter cake was washed with H2O (3 x 300 mL). The collected solid was dried to a constant weight at 5O0C under vacuum in a vacuum oven providing 345.9 g (90.1%) of [6-(4-methyl-piperazin-1-yl)-1H- benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder. In an alternative work up procedure, the filtrates were combined and the ethanol was removed under vacuum until at least about 90% had been removed. Water at a neutral pH was then added to the resulting oil, and the solution was cooled to about O0C. An aqueous 20% NaOH solution was then added EPO <DP n="59"/>slowly with rapid stirring to bring the pH up to 9.2 (read with pH meter). The resulting mixture was then filtered and dried as described above. The alternative work up procedure provided the light tan to light yellow product in yields as high as 97%. |
|
at 40 - 55℃; for 1.25 - 2.5h;Product distribution / selectivity; |
A 5000 ml_, 4-neck flask was fitted with a stirrer, thermometer, condenser, and gas inlet/outlet. The equipped flask was charged with 265.7 g (1.12 mol. 1.0 eq) of 5-(4-methyl-piperazin-1-yl)-2-nitroaniline and 2125 ml_ of 200 proof EtOH. The resulting solution was purged with N2 for 15 minutes. Next, 20.0 g of 5% Pd/C (50% H2O w/w) was added. The reaction was vigorously stirred at 40-500C (internal temperature) while H2 was bubbled through the mixture. The reaction was monitored hourly for the disappearance of 5-(4- methyl-piperazin-1-yl)-2-nitroaniline by HPLC. The typical reaction time was 6 hours.[0091] After all the 5-(4-methyl-piperazin-1-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. Next, 440.0 g (2.25 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added as a solid. The reaction was stirred at 40-50C (internal temperature) until the reaction was complete. The reaction was monitored by following the disappearance of the diamino compound by HPLC. The typical reaction time was <n="31"/>1-2 hours. After the reaction was complete, it was cooled to room temperature and filtered through a pad of Celite filtering material. The Celite filtering material was washed with absolute EtOH (2 x 250 mL), and the filtrate was concentrated under reduced pressure providing a thick brown/orange oil. The resulting oil was taken up in 850 mL of a 0.37% HCI solution. Solid NaOH (25 g) was then added in one portion, and a precipitate formed. The resulting mixture was stirred for 1 hour and then filtered. The solid was washed with H2O (2 x 400 mL) and dried at 50C in a vacuum oven providing 251.7 g (74.1 %) of [6-(4-methyl-piperazin-1-yl)- 1 H-benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder.; A 5000 mL, 4-neck jacketed flask was fitted with a mechanical stirrer, condenser, temperature probe, gas inlet, and oil bubbler. The equipped flask was charged with 300 g (1.27 mol) of 5-(4-methyl-piperazin-1-yl)-2- nitroaniline and 2400 mL of 200 proof EtOH (the reaction may be and has been conducted with 95% ethanol and it is not necessary to use 200 proof ethanol for this reaction). The resulting solution was stirred and purged with N2 for 15 minutes. Next, 22.7 g of 5% Pd/C (50% H2O w/w) was added to the reaction flask. The reaction vessel was purged with N2 for 15 minutes. After purging with N2, the reaction vessel was purged with H2 by maintaining a slow, but constant flow of H2 through the flask. The reaction was stirred at 45-55C (internal temperature) while H2 was bubbled through the mixture until the 5-(4-methyl- piperazin-1-yl)-2-nitroaniline was completely consumed as determined by HPLC. The typical reaction time was 6 hours.[0093] After all the 5-(4-methyl-piperazin-1-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. The diamine intermediate is air sensitive so care was taken to avoid exposure to air. 500 g (2.56 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added to the reaction mixture over a period of about 30 minutes. The reaction was stirred at 45-550C (internal temperature) under N2 until the diamine was completely consumed as determined by HPLC. The typical reaction time was <n="32"/>about 2 hours. After the reaction was complete, the reaction was filtered while warm through a pad of Celite. The reaction flask and Celite were then washed with 200 proof EtOH (3 x 285 ml_). The filtrates were combined in a 5000 mL flask, and about 3300 ml_ of ethanol was removed under vacuum producing an orange oil. Water (530 mL) and then 1 M HCL (350 ml_) were added to the resulting oil, and the resulting mixture was stirred. The resulting solution was vigorously stirred while 30% NaOH (200 mL) was added over a period of about 20 minutes maintaining the internal temperature at about 25-30C while the pH was brought to between 9 and 10. The resulting suspension was stirred for about 4 hours while maintaining the internal temperature at about 20-250C. The resulting mixture was filtered, and the filter cake was washed with H2O (3 x 300 mL). The collected solid was dried to a constant weight at 50C under vacuum in a vacuum oven providing 345.9 g (90.1%) of [6-(4-methyl-piperazin-1-yl)-1 H- benzoimidazol-2-yl]-acetic acid ethyl ester as a pale yellow powder. In an alternative work up procedure, the filtrates were combined and the ethanol was removed under vacuum until at least about 90% had been removed. Water at a neutral pH was then added to the resulting oil, and the solution was cooled to about 0C. An aqueous 20% NaOH solution was then added slowly with rapid stirring to bring the pH up to 9.2 (read with pH meter). The resulting mixture was then filtered and dried as described above. The alternative work up procedure provided the light tan to light yellow product in yields as high as 97%. |
|
In ethanol; water; at 40 - 55℃; for 1 - 2h;Product distribution / selectivity; |
After all the 5- (4-methyl-piperazin-1-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. Next, 440.0 g (2.25 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added as a solid. The reaction was stirred at 40-50C (internal temperature) until the reaction was complete. The reaction was monitored by following the disappearance of the diamino compound by HPLC. The typical reaction time was 1-2 hours. After the reaction was complete, it was cooled to room temperature and filtered through a pad of Celite filtering material. The Celite filtering material was washed with absolute EtOH (2 x 250 mL), and the filtrate was concentrated under reduced pressure providing a thick brown/orange oil. The resulting oil was taken up in 850 mL of a 0.37% HC1 solution. Solid NaOH (25 g) was then added in one portion, and a precipitate formed. The resulting mixture was stirred for 1 hour and then filtered. The solid was washed with H20 (2 x 400 mL) and dried at 50C in a vacuum oven providing 251.7 g (74.1%) of [6- (4-methyl-piperazin-1-yl)-lH-benzoimidazol-2-yl]- acetic acid ethyl ester as a pale yellow powder.After all the 5- (4-methyl-piperazin-1-yl)-2-nitroaniline had disappeared from the reaction, the solution was purged with N2 for 15 minutes. The diamine intermediate is air sensitive so care was taken to avoid exposure to air. 500 g (2.56 mol) of ethyl 3-ethoxy-3-iminopropanoate hydrochloride was added to the reaction mixture over a period of about 30 minutes. The reaction was stirred at 45- 55C (internal temperature) under N2 until the diamine was completely consumed as determined by HPLC. The typical reaction time was about 2 hours. After the reaction was complete, the reaction was filtered while warm through a pad of Celite. The reaction flask and Celite were then washed with 200 proof EtOH (3 x 285 mL). The filtrates were combined in a 5000 mL flask, and about 3300 mL of ethanol was removed under vacuum producing an orange oil. Water (530 mL) and then 1M HCL (350 mL) were added to the resulting oil, and the resulting mixture was stirred. The resulting solution was vigorously stirred while 30% NaOH (200 mL) was added over a period of about 20 minutes maintaining the internal temperature at about 25-30C while the pH was brought to between 9 and 10. The resulting suspension was stirred for about 4 hours while maintaining the internal temperature at about 20-25C. The resulting mixture was filtered, and the filter cake was washed with H20 (3 x 300 mL). The collected solid was dried to a constant weight at 50C under vacuum in a vacuum oven providing 345.9 g (90. 1%) of [6- (4-methyl-piperazin-1-yl)-lH-benzoimidazol-2- yl] -acetic acid ethyl ester as a pale yellow powder. In an alternative work up procedure, the filtrates were combined and the ethanol was removed under vacuum until at least about 90% had been removed. Water at a neutral pH was then added to the resulting oil, and the solution was cooled to about 0C. An aqueous 20% NaOH solution was then added slowly with rapid stirring to bring the pH up to 9.2 (read with pH meter). The resulting mixture was then filtered and dried as described above. The alternative work up procedure provided the light tan to light yellow product in yields as high as 97%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping