| 98% |
With chlorosulfonic acid; In chloroform; at -7 - 20℃; for 1.5 - 1.83333h;Product distribution / selectivity; |
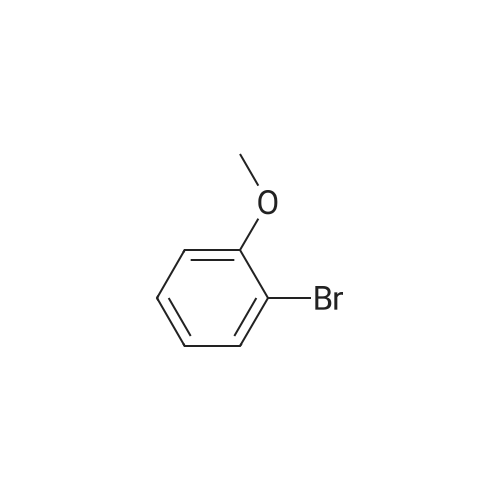
o-Bromoanisole (1.87 g, 10. OMMOL) was dissolved in chloroform (5 mL) and the solution was cooled in an ice-salt bath to-5 C to 0 C. The solution was carefully charged with CHLOROSULFONIC acid (2.0 mL, 30.0 mmol) over 30 minutes and then allowed to warm to room temperature over 1 hour. The mixture was poured onto chopped ice and transferred to separatory funnel. The organic layer was separated and the aqueous layer was extracted, dried and concentrated to give 3-bromo-4-methoxyphenylsulfonyl chloride (2.80 g, 98%).; REFERENCE 17 N-TERT-BUTYL-4-METHOXY-3- (4, 4,5, 5-TETRAMETHYL- [1, 3,2] DIOXABOROLAN-2-YL) - BENZENESULFONAMIDE [0214] A 500 mL 3-necked flask, equipped with thermometer, overhead stirrer and a 60 mL DROPPING FUNNEL, WAS CHARGED WITH 2-BROMOANISOLE (46.8 G, 0.25 MOL, 1.0 EQ. ) AND ANHYDROUS chloroform (250 mL). The flask was flushed with nitrogen and cooled with a brine-ice cool- bath to an internal temperature of-7C and then chlorosulfonic acid (87.4 g, 0.75 mol, 3.0 EQ.) was added via dropping funnel over 1 hour while maintaining an internal temperature of less THAN-5C. The reaction mixture was stirred for 50 minutes and then poured on to ice (500 g). The mixture was stirred until the ice melted and then the organic layer was separated and washed with water (2 x 200 mL). The combined aqueous layers were backwashed with chloroform (2 x 200 mL) and the combined organic phases were washed with brine and dried (MGS04). [0215] The organic phase was treated at room temperature with triethylamine (87 mL, 63 g, 0.625 MOL, 2.5 EQ. ) AND THEN TERT-BUTYLAMINE (34 ML, 24 G, 0.325 MOL, 1.3 EQ. ). THE REACTION mixture was stirred overnight, cooled in an ice-bath and poured into ice cold 2M hydrochloric acid (500 mL). The organic layer was separated and washed with 2M hydrochloric acid (2 x 250 mL) and brine, dried (MGSO4) and concentrated. Crystallization of the residue from chloroform-hexane gave 3-BROMO-N-TERT-BUTYL-4-METHOXY-BENZENESULFONAMIDE (37.5 g, 47%) as brilliant white crystals. RP-HPLC (10-95S) RT = 3.92 MIN.'H NMR (400 MHz, d6- DMSO): 8 7. 94 (1H, d, J = 2. 4 HZ), 7.77 (1H, dd, J = 8. 8,2. 4 HZ), 7.48 (1H, s), 7.25 (1H, d, 8.8 Hz), 3.92 (3H, s), 1.08 (9H, s). [0216] A mixture OF 3-BROMO-N-TERT-BUTYL-4-METHOXY-BENZENESULFONAMIDE (36.2 g, 112 mmol, 1.0 eq), bis (pinocalto) diborane (30.0 g, 117 mmol, 1.05 EQ.), potassium acetate (33.0 G, 336 MMOL, 3.0 EQ. ) AND PDCL2 (DPPF)-DCM (533 MG, 0.653 MMOL, 5.8 MOL %, IN 115 ML of 1,4-dioxane) was heated at 100 C under nitrogen and then 4,4, 5,5, 4', 4', 5', 5'- OCTAMETHYL- [2, 2'] BI [ [1, 3, 2] DIOXABOROLANYL] (0.35 EQ. ) WAS ADDED TO THE MIXTURE. THE REACTION mixture was heated for 28 hours and then allowed to cool. The mixture was filtered of solids and concentrated. The residue was dissolved in ethyl acetate (500 mL) and the solution was washed with 5% citric acid (3x 200 mL), saturated sodium bicarbonate (3 x 200 mL) and then brine, dried (MGSO4) and concentrated. Purification of product from the residue by silica-gel chromatography, eluting with 10-50% EtOAc/Hex gave N-tert-butyl-4-methoxy-3- (4, 4,5, 5- TETRAMETHYL- [1, 3,2] DIOXABOROLAN-2-YL) -BENZENESULFONAMIDE (30 G, 72%) AS A PALE-ORANGE solid. RP-HPLC (10-95S): RT = 3.17 min.'HNMR (400MHz, d6-DMSO) : 57. 95 (1H, d, J = 2.4 Hz), 7.85 (1H, dd, J = 8.8, 2.4 Hz), 7.36 (1H, s), 7.11 (1H, d, J = 8.8), 3.81 (3H, s), 1.29 (12H, s), 1.07 (9H, s); M/Z (LCMS-ESI): Q+ 310 (boronic acid+Na), 370 (M+H), 392 (M+Na); Q~ 354 (M-Me), 556 (boronic acid anhydride). |
| 97% |
With chlorosulfonic acid; In dichloromethane; at 0 - 20℃;Cooling with ice; |
A solution of 2-bromoanisole (5.00 g, 26.7 mmol) in CH2Cl2 (100 mL) was cooled on ice. Chlorosulfonic acid (9.3 g, 80 mmol) in CH2Cl2 (100 mL) was added dropwise at 0 C. The reaction mixture was allowed to reach room temperature overnight and was then added slowly to a stirred solution of brine. The organic phase was separated and washed with brine, dried over MgSO4, filtered and concentrated. The intermediate 3-bromo-4-methoxybenzenesulfonyl chloride was obtained in 97% yield (7.33 g). 1H NMR (600 MHz, CDCl3) delta ppm 4.03 (s, 3H) 7.04 (d, J=8.85 Hz, 1H) 7.99 (dd, J=8.85, 2.44 Hz, 1H) 8.22 (d, J=2.44 Hz, 1H). MS (ESI+) m/z 249 [M-Cl]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +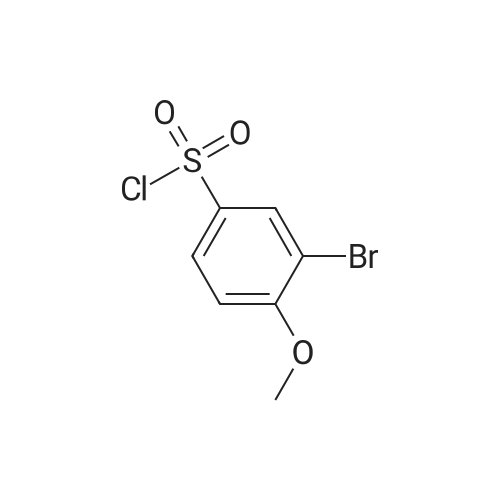

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping