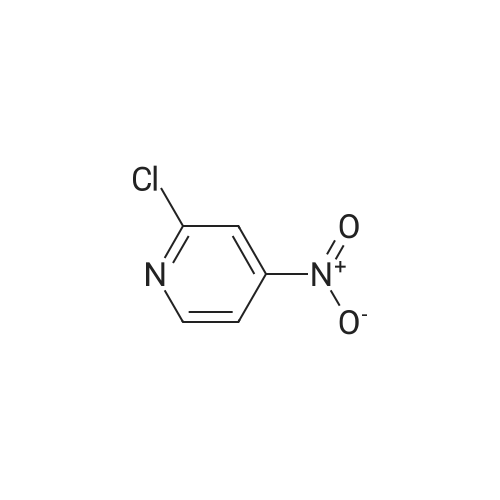
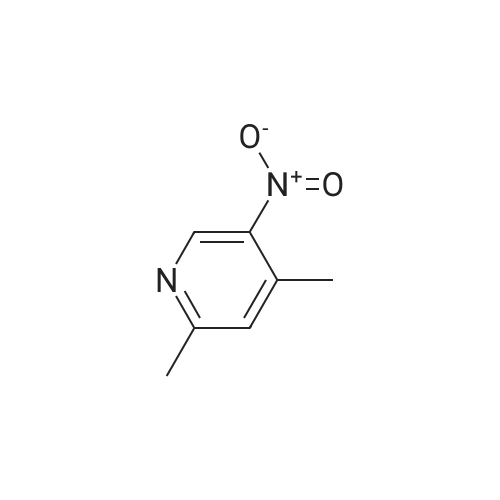
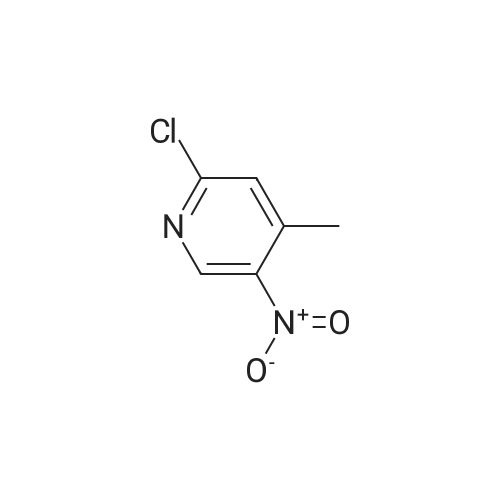
| 73% |
With chromium(VI) oxide; sulfuric acid; at 0 - 20℃; for 1h;Inert atmosphere; |
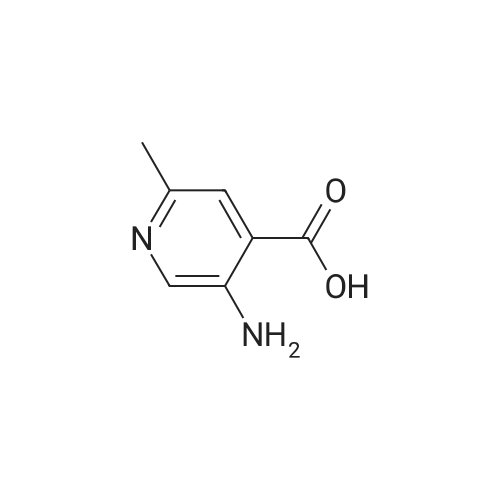
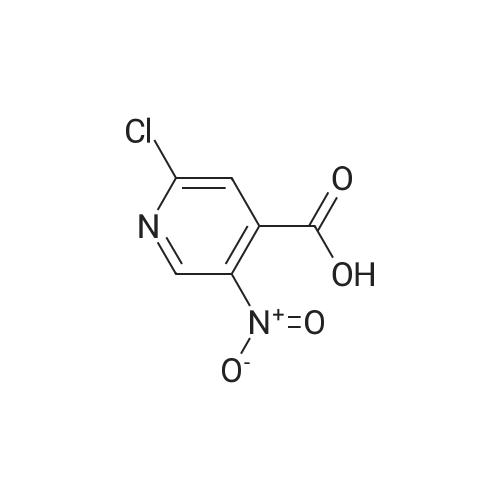
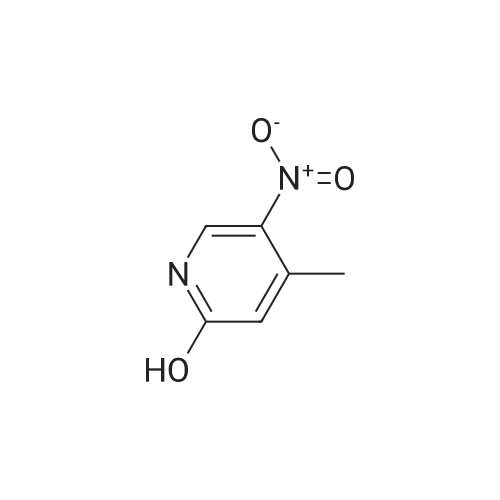
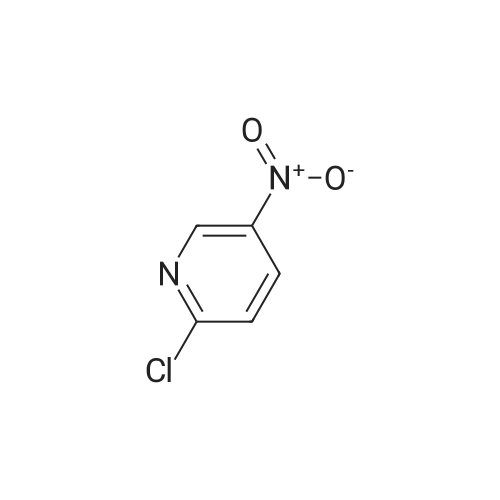
[00610] Step 1: To a solution of 2-chloro-4-methyl-5-nitro-pyridine (10.35 g, 60 mmol) in H2S04 (100 mL), Cr03 (19.8 g, 198 mmol) was added at 0 C. The mixture was stirred at 0 C for 1 h and then was allowed to warm to room temperature and stirred overnight. The mixture was poured into ice water (500 mL). The resulting solid was filtered and dried to give 2-chloro-5-nitro-isonicotinic acid (8.89 g, yield: 73%) as a white solid. MS: m/z 200.9 (M-H+). |
|
With chromium(VI) oxide; sulfuric acid; at 0 - 20℃; |
A solution of 2-chloro-4-methyl-5-nitropyridine (5.13 g, 29.73 mmol) in concentrated sulfuric acid (42 mL) was cooled to 0 C., and chromium trioxide (9.81 g, 98.1 mmol) was added. The mixture was stirred at 0 C. for 1 hour and then warmed to room temperature, with an oil bubbler attached, for overnight stirring. The reaction mixture was poured onto ice (300 ml) and diluted with water (150 ml). The mixture was warmed to room temperature, and the solid was filtered and then dried under vacuum to yield 2-chloro-5-nitroisonicotinic acid. To a stirred solution of the above material (5.3 g, 26.17 mmol) in methanol (50 ml) was added chloroform (200 ml). TMS-diazomethane as a solution in hexane (2M) was added dropwise until the color of the reaction mixture remained yellow (20 mL). The residual TMS-diazomethane was quenched by addition of acetic acid, and the solvent was removed under reduced pressure. The oily residue was subjected to silica gel chromatography eluted with 50-70% ethyl acetate in hexane to provide methyl 2-chloro-5-nitroisonicotinate. A solution of the above material (5.66 g, 26.13 mmol), methyl 4'-(aminomethyl)-3,3'-difluorobiphenyl-2-carboxylate (7.971 g, 28.75 mmol, prepared according to procedures described in WO 03/066577), and triethylamine (3.97 g, 39.20 mmol) in methanol (100 ml) was stirred at room temperature overnight. The solution was then heated at 60 C. for 4 hours and cooled to ambient temperature for continued stirring over the weekend. Solvent was removed, and the residue was subjected to silica gel chromatography eluted with 25-50% ethyl acetate in hexane to provide methyl 2-([3,3'-difluoro-2'-(methoxycarbonyl)biphenyl-4-yl]methyl}amino)-5-nitroisonicotinate as a yellow solid. A solution of the above material (9.3 g, 20.33 mmol) in methanol (330 ml) was purged with nitrogen, and 10% Pd/C catalyst (1 g) was added. The reaction vessel was again purged with nitrogen and then with hydrogen from a balloon. After 23 hours of stirring under hydrogen, nitrogen was bubbled through the solution for 10 minutes prior to filtration through a celite pad. The filtrate was concentrated under reduced pressure to provide methyl 5-amino-2-([3,3'-difluoro-2'-(methoxycarbonyl)biphenyl-4-yl]methyl}amino)isonicotinate. Into a solution of the above material (8.45 g, 19.77 mmol) in THF (440 ml) at 0 C. were added hypophosphorous acid (50% solution in water, 110 ml) and sodium nitrite (2.73 g, 39.54 mmol). After 10 minutes of stirring, a catalytic amount of copper (I) oxide was added every 30 minutes for 7.5 hours. The reaction mixture was partitioned between ethyl acetate and water, and the aqueous layer was extracted with additional ethyl acetate. The combined organic layers were washed with saturated sodium bicarbonate and brine, then dried over sodium sulfate, filtered, and concentrated under reduced pressure. The residue was subjected to silica gel chromatography eluted with 20-40% ethyl acetate in hexane to provide methyl 2-([3,3'-difluoro-2'-(methoxycarbonyl)biphenyl-4-yl]methyl}amino)isonicotinate. To a stirred solution of the above material (3.96 g, 9.60 mmol) in methanol (85 ml) was added 1N NaOH (11.5 ml), and the solution was heated at 40 C. for 3.5 hours. Solvent was removed under reduced pressure prior to dilution with water. The aqueous solution was washed with diethyl ether twice, and the residual diethyl ether in the aqueous solution was removed under reduced pressure. The aqueous solution was neutralized by addition of 1N HCl (11.5 ml), and the resulting thick suspension was heated (70 C.) and then slowly cooled to ambient temperature before being cooled to 0 C. for 30 minutes. The solid was filtered and dried under vacuum, providing the title compound as a white solid. LRMS (ES, M+H+): 399. 1H NMR (CD3OD, 400 MHz) delta 8.04 (d, J=5.6 Hz, 1H), 7.55 (m, 1H), 7.44 (t, J=8 Hz, 1H), 7.23 (m, 3H), 7.10 (m, 3H), 4.65 (s, 2H), 3.66 (s, 3H). |
|
With chromium(VI) oxide; sulfuric acid; at 0 - 20℃; |
EXAMPLE 10 Methyl 3,3,'-difluoro-4'-([2-(4-pyridin-4-ylpiperazin-1-yl)isonicotinoyl]amino}-methyl)biphenyl-2-carboxylate Commercially available 2-chloro-4-methyl-5-nitropyridine (5.13 g, 29.73 mmol) was dissolved in 42 ml conc. sulfuric acid. The resulting solution was cooled to 0 C., and chromium trioxide (9.81 g, 98.1 mmol) was added to the solution. After 1 hour of stirring at 0 C., the mixture was warmed to room temperature with a bubbler attached to the flask. After overnight stirring, the reaction mixture was poured onto ice (300 ml) and diluted with water (150 ml). The mixture was allowed to warm to room temperature and the solid was filtered and dried under vacuum overnight to get 2-chloro-5-nitroisonicotinic acid as a light green solid. |
|
With sodium dichromate; sulfuric acid; at 15 - 20℃;Cooling with ice; |
Intermediate 25A: 2-Chloro-5-nitroisonicotinic acid 2-chloro-4-methyl-5-nitropyridine (1 .0 g, 5.8 mmol) was cooled using an ice water bath. Sodium dichromate dihydrate (3.45 g, 1 1 .6 mmol) dissolved in sulphuric acid (50 mL) was added dropwise, ensuring reaction temperature does not exceed 15C. After complete addition, reaction allowed to warm to room temperature and stirred overnight. Quenched with ice, extracted into ethylacetate and concentrated under reduced pressure to afford 2- chloro-5-nitroisonicotinic acid (1.3 g, 6.4 mmol). |
|
With sodium dichromate; sulfuric acid; at 60℃; for 6h; |
Synthesis of the compound 33 The compound 32 (9.45 g, 0.055 mol) was dissolved in a concentrated sulphuric acid (80mL) with agitation, a sodium dichromate (19.2 g, 0.065 mol) was added slowly in batches into the system, and the reaction was run at 60C for 6 h. The above reaction liquid was added slowly into broken ice (250 g) and extracted with ethyl acetate (300 mL) three times. The extracts were combined and washed with a saturated aqueous solution of table salt. The solvent was evaporated off and 8.65 g of the crude product was obtained with a yield of 77.6%. The crude product was recrystallized in a mixture of ethanol and petroleum ether (1:2) to obtain a white solid product with the melting point of 193.3-193.6C (ethanol/petroleum ether). |
| 18 g |
With chromium(VI) oxide; sulfuric acid; at 0 - 20℃; for 13h; |
2-Chloro-4-methyl-5-nitropyridine (20.5 g, 119 mmol) was dissolved in concentrated sulfuric acid (200 ml), and chromium trioxide (40.0 g, 400 mmol) was added thereto, followed by stirring at 0 C. for 1 hour. Then, the temperature was gradually raised from 0 C. to room temperature, followed by stirring for 12 hours. The reaction solution was poured into ice-water (2000 ml), and the temperature was raised from 0 C. to room temperature. The precipitated solid was filtered and dried under reduced pressure to obtain the title compound (18 g, 750). [0144] 1H NMR (CD3OD, 400 MHz): delta 10.8 (1H, br, s), 9.13 (1H, s), 7.70 (1H, s) |
|
With potassium dichromate; sulfuric acid; at 60℃; for 14h; |
To a solution of 2-chloro-4-methyl-5-nitropyridine (46 g, 267 mmol) in H2SO4(400 ml) was added K2Cr2O7(98 g, 333 mmol) in several portions at 60 . The resulting mixture was stirred at the same temperature for 14 h to give a dark green solution. After cooling, the mixture was poured into ice (250 g) carefully and the aqueous was extracted with EtOAc (500 ml*3) . The organic phase was concentrated under reduced pressure to give the product as a solid which was used in next step without further purification. MS (ESI) calcd. for (C6H4ClN2O4) [M+H] +, 203.0, found, 203.0 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping