| 65% |
With thionyl chloride; triethylamine; In dichloromethane; |
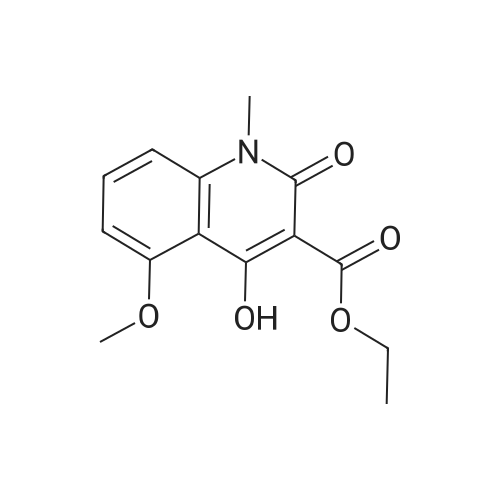
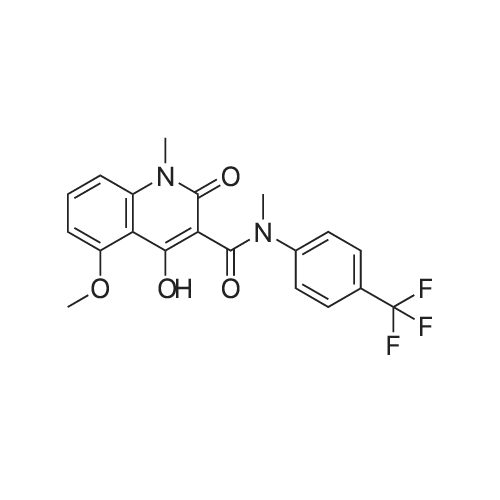
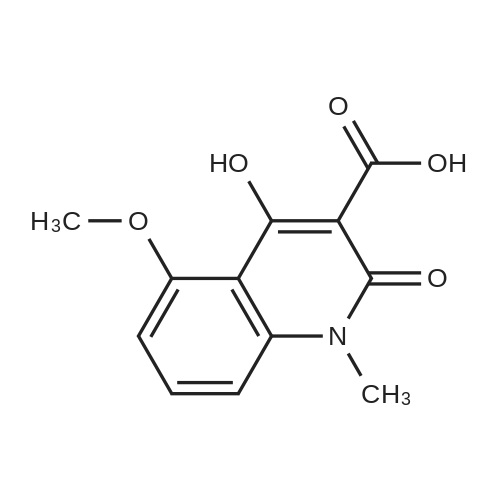
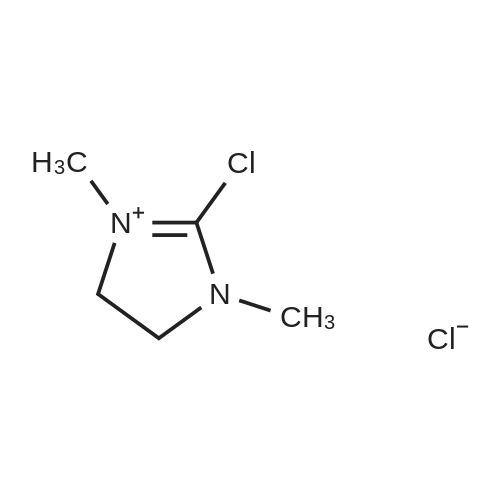
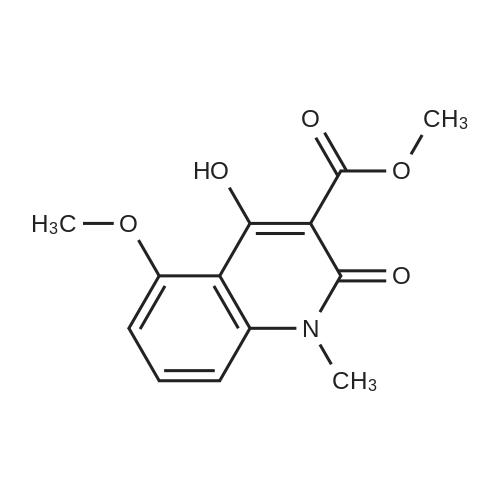
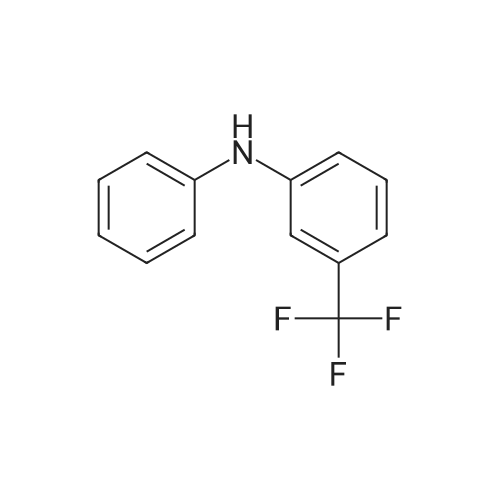
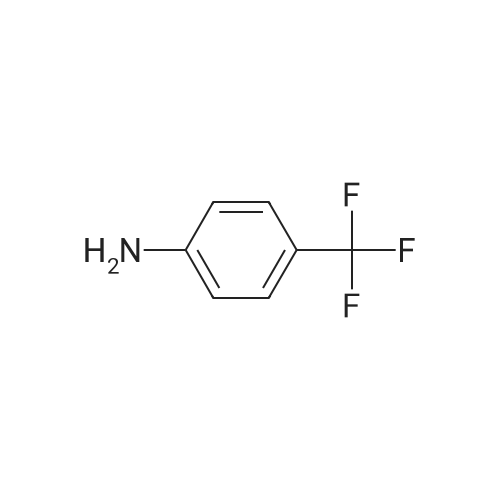
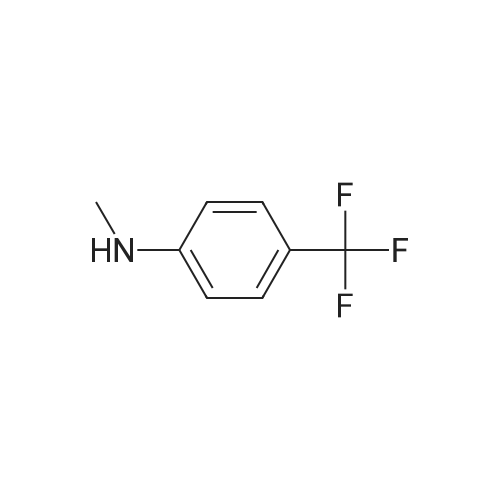
EXAMPLE 8 N-Methyl-N-(4-trifluoromethyl-phenyl)-1,2-dihydro-4-hydroxy-5-methoxy-1-methyl-2-oxo-quinoline-3-carboxamide (Method B) To an ice-cold solution of 1,2-dihydro-4-hydroxy-5-methoxy-1-methyl-2-oxo-quinoline-3-carboxylic acid (8 g, 0.032 mol), triethylamine (15.5 ml, 0.11 mol) and 4-trifluoromethyl-N-methylaniline (6.1 g, 0.035 mol) in 150 ml of methylene chloride was added dropwise during 0.5 hours a solution of thionyl chloride (3.0 ml, 0.042 mol) in 10 ml of methylene chloride. The stirring was continued at 4° C. for 4 hours. The solution was diluted with 10 ml of methylene chloride, washed with cold 1 M sulphuric acid and then extracted with 1 M sodium hydroxide. The pH of the aqueous phase was adjusted to 8-8.5, clarified by filtration and then acidified with hydrochloric acid to pH 4. On standing a crystalline precipitate was formed which was filtered off, washed with water and dried to give the title compound (8.5 g) yield 65percent. 1H NMR (CDCl3) delta 3.48 (3H, s), 3.54 (3H, s), 4.06 (3H, s), 6.70 (1H, d), 6.94 (1H, d), 7.46 (1H, t), 7.50 (4H, broad signal). 13C NMR (CDCl3) delta 29.8 (CH3), 36.9 (CH3), 56.9 (CH3), 103.5 (CH), 104.2 (C), 108.7 (CH), 109.5 (C), 117.3+121.7+126.0+130.3 (C), 125.8+125.9+125.9+126.0 (CH), 126.3 (CH), 127.9+128.4+128.9+129.4 (C), 131.8 (CH), 141.4 (C), 146.7 (C), 157.2 (C), 158.0 (C), 160.3 (C), 165.0 (C); some peaks are multiplets due to F-coupling. ESI MS/MS [M+H]+ 407, fragment 232. |
| 65% |
With thionyl chloride; triethylamine; In dichloromethane; at 0 - 4℃; for 4.5h; |
To an ice-cold solution of 1,2-dihydro-4-hydroxy-5-methoxy-1-methyl-2-oxo-quinoline-3-carboxylic acid (8 g, 0.032 mol), triethylamine (15.5 ml, 0.11 mol) and 4-trifluoromethyl-N-methylaniline (6.1 g, 0.035 mol) in 150 ml of methylene chloride was added dropwise during 0.5 hours a solution of thionyl chloride (3.0 ml, 0.042 mol) in 10 ml of methylene chloride. The stirring was continued at 4°C for 4 hours. The solution was diluted with 10 ml of methylene chloride, washed with cold 1 M sulphuric acid and then extracted with 1 M sodium hydroxide. The pH of the aqueous phase was adjusted to 8-8.5, clarified by filtration and then acidified with hydrochloric acid to pH 4. On standing a crystalline precipitate was formed which was filtered off, washed with water and dried to give the title compound (8.5 g) yield 65 percent. 1H NMR (CDCl3) delta 3.48 (3H, s), 3.54 (3H, s), 4,06 (3H, s), 6.70 (1H, d), 6.94 (1H, d), 7.46 (1H, t), 7.50 (4H, broad signal). 13C NMR (CDCl3) delta 29.8 (CH3), 36.9 (CH3), 56.9 (CH3), 103.5 (CH), 104.2 (C), 108.7 (CH), 109.5 (C), 117.3+121.7+126.0+130.3 (C), 125.8+125.9+125.9+126.0 (CH), 126.3 (CH), 127.9+128.4+128.9+129.4 (C), 131.8 (CH), 141.4 (C), 146.7 (C), 157.2 (C), 158.0 (C), 160.3 (C), 165,0 (C); some peaks are multiplets due to F-coupling. ESI MS/MS [M+H]+ 407, fragment 232. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping