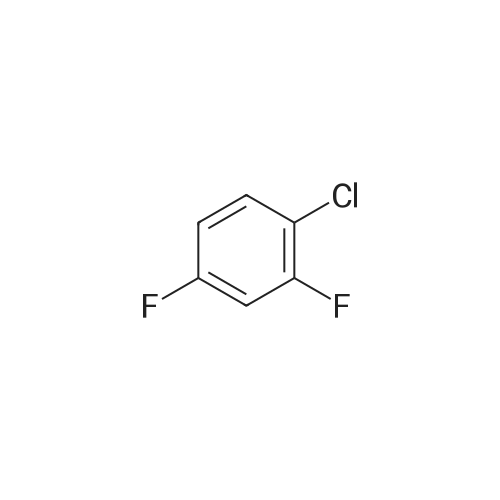
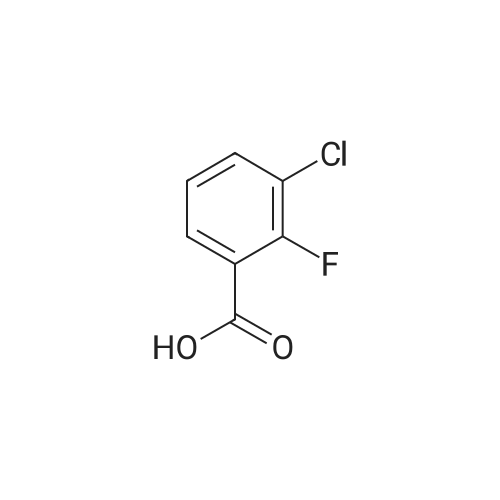
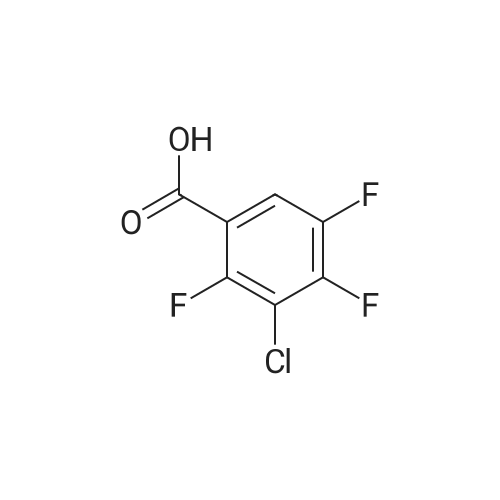
|
|
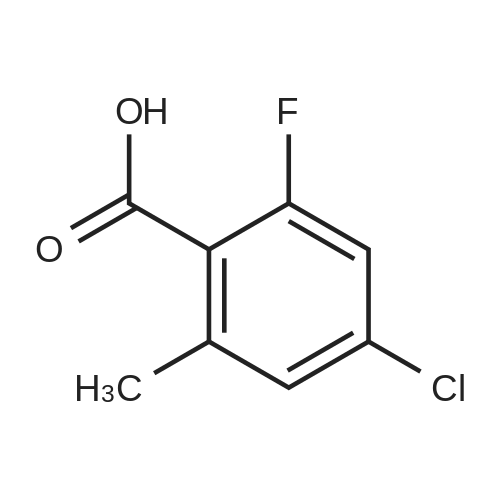
Intermediate 5 1 -(3-Chloro-2,6-difluoro henyl)- 1H- 1 ,2,3-triazole-4-carboxylic acid [00325] Intermediate 5A: tert-Butyl (3-chloro-2,6-difluorophenyl)carbamate: 3- chloro-2,6-difluorobenzoic acid (4.85 g, 25.2 mmol) was dissolved in THF (50 mL) and cooled to 0 C. To this solution was then added ethylchloroformate (3.01 g, 27.7 mmol) followed by TEA (3.86 mL, 27.7 mmol) and stirred at the same temperature for 1 h. To the slurry that developed was then added NaN3 in H20 (5 mL) dropwise and stirred the reaction mixture at 0 C for 1.25 h. Solids separated out from the reaction mixture and allowed the solids to decant followed by separation of the decantant. The residue was dissolved in H20 (50 mL) and extracted with DCM (2x). The above organic layer was then combined with the decantant, dried (MgS04) and concentrated to yield a residue. - I l l - The residue was re-dissolved in toluene (50 mL) and heated at 110 C. To the above solution was added t-BuOH (1.5 g) and refluxed for overnight. The reaction mixture was concentrated and purified by silica gel chromatography to yield the desired product (2.86 g, 43%). MS(ESI) m/z: 286.0 (M+Na)+. 1H NMR (400 MHz, CDC13) delta 7.28 (s, 1H), 7.03 - 6.72 (m, 1H), 6.10 - 5.83 (m, 1H), 1.57 - 1.37 (m, 9H) ppm. |
|
|
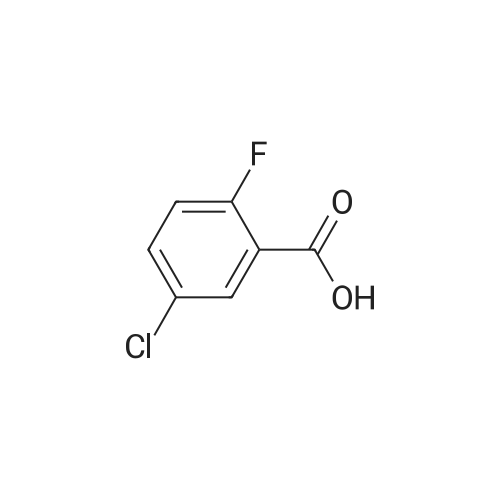
[0119] <strong>[225104-76-7]3-chloro-2,6-difluorobenzoic acid</strong> (0.08 g, 0.41 mmol) was dispersed in diethyl ether (5.2 mL), slowly addedwith phosphorus pentachloride (PCl5, 0.099 g, 0.48 mmol), and then stirred for 1 hour. Upon completion of the reaction,the organic solvent was concentrated under reduced pressure below room temperature, and then the reaction solutionwas diluted by adding acetone (3.5 mL). Subsequently, sodium azide (NaN3, 0.032 g, 0.50 mmol) dissolved in water(0.25 mL) was slowly added to the reaction solution dropwise at 0C. After stirring the reaction solution for 2 hours atroom temperature, 3-chloro-2,6-difluorobenzoyl azide thus formed was diluted with ethyl acetate, followed by washingwith water. The organic layer was dried over anhydrous magnesium sulfate, dispersed in THF (1.6 mL), added with THF(1.6 mL) containing 4-(4-amino-2-fluorophenyl)-7-(5-methyl-1H-imidazol-2-yl)isoindolin-1-one (Compound D, 0.067 g,0.21 mmol), and then stirred for 3 hours at 90C. Upon completion of the reaction, the solvent was concentrated, andthen purified by silica gel column chromatography (eluent: methylene chloride : methanol = 20:1) to obtain the titlecompound (0.017 g, yield: 16%).[0120] 1H-NMR Spectrum(300 MHz, DMSO-d6): 14.47-14.38 (m, 1H), 9.49-9.39 (m, 2H), 8.53 (s, 1H), 8.44 (d, J=8.1Hz,1H), 7.63-7.47 (m, 4H), 7.31-7.24 (m, 2H), 7.09-6.84 (m, 1H), 4.42 (s, 2H), 2.31-2.21 (m, 3H)[0121] LCMS [M+1]: 512.3 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping