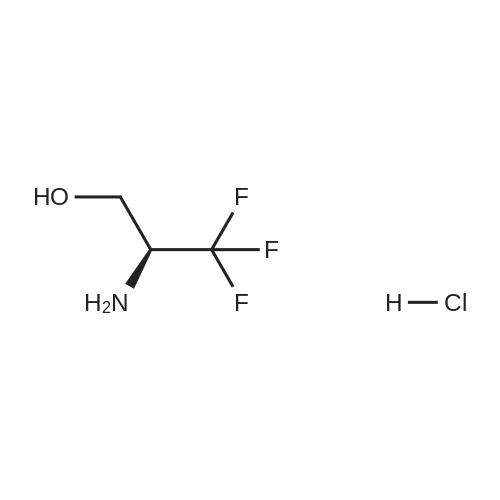
| 71% |
With sodium hydride; In 1,4-dioxane; at 0 - 90℃; for 2h;Inert atmosphere; |
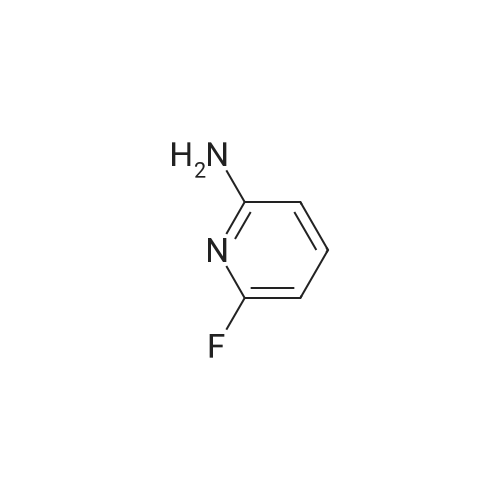
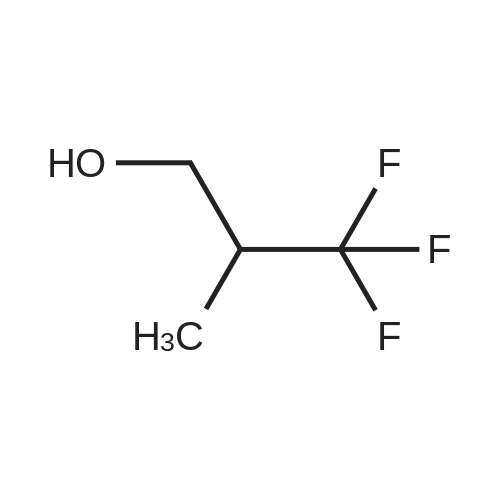
A mixture of <strong>[1597-32-6]6-fluoropyridin-2-amine</strong> (1.00 g, 8.92 mmol) and 3,3,3-trifluoropropan-1-ol (2.03 g, 17.84 mmol) in dioxane (15 mL) was treated with NaH (0.86 g, 35.7 mmol) at 0 C under nitrogen atmosphere and then heated at 90 C for 2 h. The reaction mixture was quenched with cold water and extracted with EtOAc. The organic extract was washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The crude material was purified by flash column chromatography eluting with 10 % to 20 % EtOAc in petroleum ether to provide the title compound (1.30 g, 6.31 mmol, 71 % yield) as a light-yellow oil. 1H NMR (400 MHz, DMSO-d6): d ppm 7.29 (t, J=7.8 Hz, 1 H), 6.01 (dd, J=7.8, 0.7 Hz, 1 H), 5.80 - 5.91 (m, 3 H), 4.34 (t, J=6.2 Hz, 2 H), 2.74 (dtd, J=17.7, 11.6, 6.2 Hz, 2 H). m/z (ESI): 207.2 (M+H)+. |
| 50% |
With sodium hydride; In 1,4-dioxane; mineral oil; at 0 - 90℃; for 2h;Inert atmosphere; |
To a solution of <strong>[1597-32-6]6-fluoropyridin-2-amine</strong> (50 g, 450 mmol, Combi-Blocks) in 1,4-dioxane (500 mL) was added 3,3,3-trifluoropropan-1-ol (102 g, 892 mmol, Apollo) under nitrogen atmosphere and the reaction was cooled to 0 C. NaH (60% in mineral oil, 42.8 g, 1780 mmol) was added to the reaction mixture at 0 C and the resulting mixture was stirred at 90 C for 2 h. The reaction mixture was quenched with cold water (500 mL) and extracted with ethyl acetate (2 x 1000 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated under reduced pressure. The crude residue was purified by column chromatography over silica gel (60-120 mesh) using 10% ethyl acetate in hexanes to give the title compound (45 g, 50 % yield) as a pale brown oil. 1H NMR (400 MHz, DMSO-d6): d 7.30- 7.26 (t, J = 7.8 Hz, 1H), 6.02- 6.00 (dd, J = 7.8, 0.8 Hz, 1H), 5.89- 5.86 (m, 3H), 4.36- 4.33 (t, J = 6.2 Hz, 2H), 2.79- 2.67 (qt, J = 11.5, 6.2 Hz, 2H). m/z (ESI): 207.1 (M+H)+. |
| 50% |
With sodium hydride; In 1,4-dioxane; mineral oil; at 0 - 90℃; for 2h;Inert atmosphere; |
To a solution of <strong>[1597-32-6]6-fluoropyridin-2-amine</strong> (50 g, 450 mmol, Combi-Blocks) in 1,4- dioxane (500 mL) was added 3,3,3-trifluoropropan-1-ol (102 g, 892 mmol, Apollo) under nitrogen atmosphere and the reaction was cooled to 0 C. NaH (60% in mineral oil, 42.8 g, 1780 mmol) was added to the reaction mixture at 0 C and the resulting mixture was stirred at 90 C for 2 h. The reaction mixture was quenched with cold water (500 mL) and extracted with ethyl acetate (2 x 1000 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated under reduced pressure. The crude residue was purified by column chromatography over silica gel (60-120 mesh) using 10% ethyl acetate in hexanes to give the title compound (45 g, 50 % yield) as a pale brown oil.1H NMR (400 MHz, DMSO-d6): 7.30 - 7.26 (t, J = 7.8 Hz, 1H), 6.02 - 6.00 (dd, J = 7.8, 0.8 Hz, 1H), 5.89 - 5.86 (m, 3H), 4.36 - 4.33 (t, J = 6.2 Hz, 2H), 2.79 - 2.67 (qt, J = 11.5, 6.2 Hz, 2H). m/z (ESI): 207.1 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +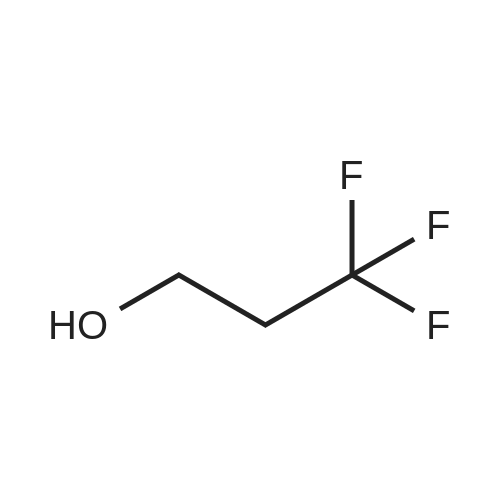

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping