Alternatived Products of [ 22398-14-7 ]
Product Details of [ 22398-14-7 ]
| CAS No. : | 22398-14-7 |
MDL No. : | MFCD00038225 |
| Formula : |
C7H10O5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | RHFZTBSULNJWEI-UHFFFAOYSA-N |
| M.W : |
174.15
|
Pubchem ID : | 90770 |
| Synonyms : |
|
Application In Synthesis of [ 22398-14-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 22398-14-7 ]
- 1
-
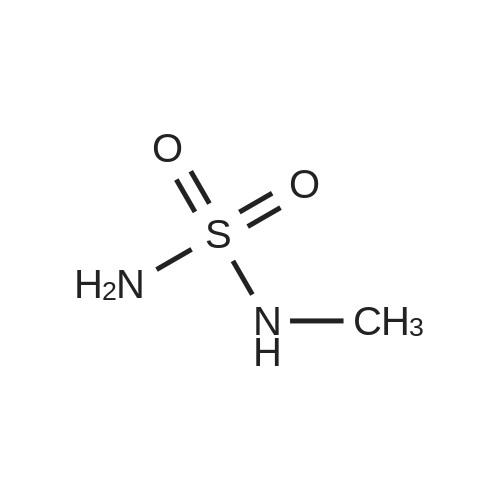 [ 72179-84-1 ]
[ 72179-84-1 ]

-
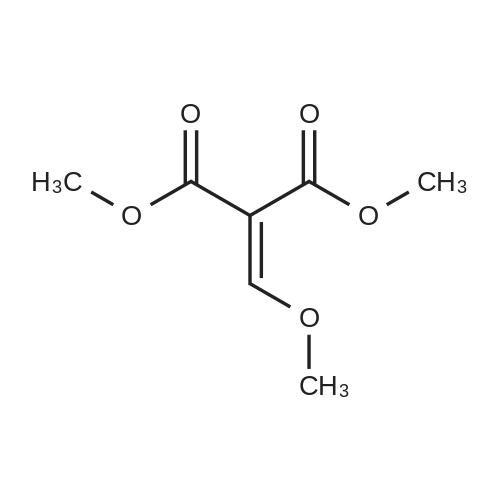 [ 22398-14-7 ]
[ 22398-14-7 ]

-
 [ 72179-86-3 ]
[ 72179-86-3 ]
- 2
-
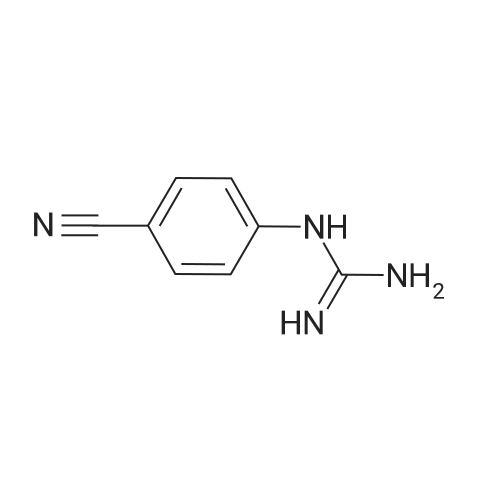 [ 5637-42-3 ]
[ 5637-42-3 ]

-
 [ 22398-14-7 ]
[ 22398-14-7 ]

-
4-[(1,4-dihydro-4-oxo-2-pyrimidinyl)amino]benzonitrile
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
Examples Example 1A mixture of 64 g (0.4 mol) of <strong>[5637-42-3](4-cyanophenyl)guanidine</strong>, 98.4 g (1.2 mol) sodium acetate and 76.6g (0.44 mol) dimethyl(methoxymethylene)malonate in 600 ml N-methylpyrrolidinone (NMP) was heated to 100C and stirred for 1 hour at that temperature. 64.8 ml of demineralized water was added and the reaction mixture was further heated to reflux temperature. About 100 ml of the solvent was evaporated until the temperature of the reaction mixture reached the range of 155C to 160C. Subsequently the reaction mixture was refluxed during 30 hours. The whole is allowed to cool to 20-25C and 25g filtration aid was added. After stirring the mixture for 1 hour at 20-25C, the precipitate was filtered off and washed with 40 ml of NMP. The solvent was distilled off under vacuum and the residue was heated to 120C. 300 ml acetic acid was added dropwise (during 15 minutes) to the heated residue while keeping the temperature at 130C. After addition of the acetic acid, the mixture was heated to 150C and stirred at that temperature for 15 minutes. Subsequently, the mixture was allowed to cool to 20-25C. The formed precipitate was filtered off and washed with ethanol (I x 200 ml and 1 x 80ml). 400ml ethanol was added to the washed precipitate and this mixture was heated and refluxed for 1 hour. After cooling to 20-25C, the precipitate was filtered off, washed with 100 ml ethanol and dried at 50C under vacuum during 16 hours. Yield : 65.6g of 4-[(l,4-dihydro-4-oxo-2-pyrimidinyl)- amino]benzonitrile. |
- 3
-
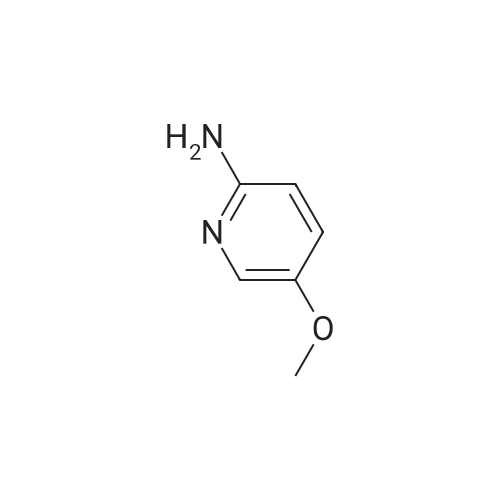 [ 10167-97-2 ]
[ 10167-97-2 ]

-
 [ 22398-14-7 ]
[ 22398-14-7 ]

-
methyl 7-methoxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-3-carboxylate
[ No CAS ]
- 4
-
 [ 10167-97-2 ]
[ 10167-97-2 ]

-
 [ 22398-14-7 ]
[ 22398-14-7 ]

-
dimethyl 2-(((5-methoxypyridin-2-yl)amino)methylene)malonate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 130℃; for 3h; |
To an oven dried flask equipped with a stir bar and air-to-air condenser was added 5- methoxypyridin-2-amine (0.2 grams, 1.61 mmol) and dimethyl 2-(methoxymethylene)malonate (0.281 grams, 1.61 mmol). The mixture was heated to 130 °C for three hours then cooled to room temperature. The solid material collected by filtration, washed with hexanes, and used without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









