| 100% |
|
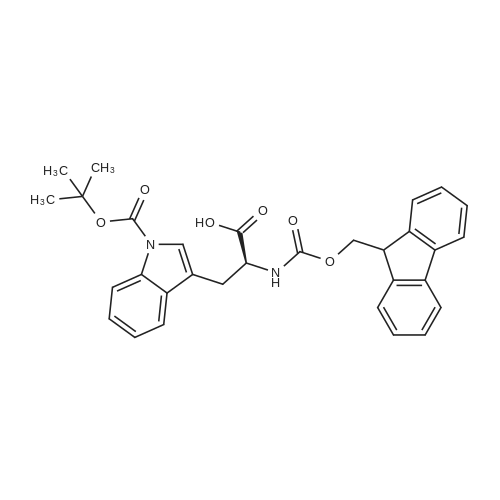
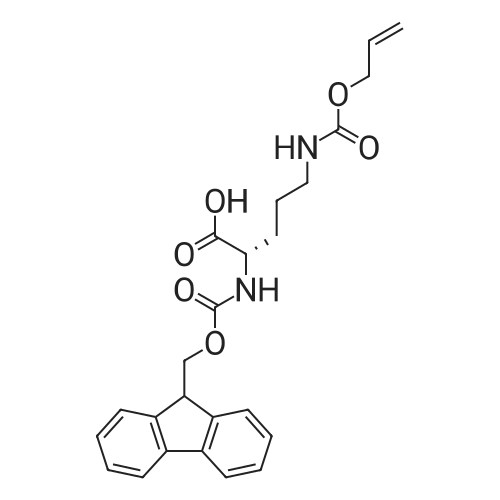
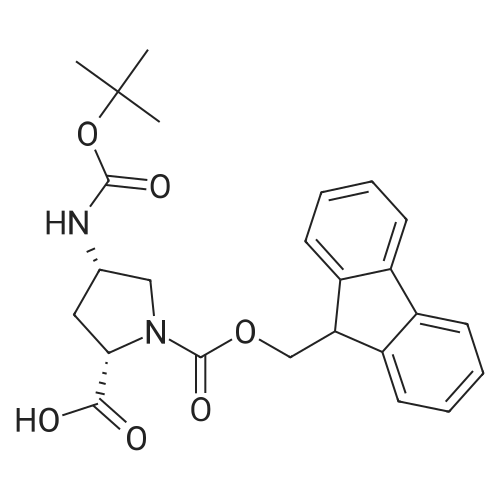
General procedure: In an SPPS vessel equipped with an overhead mechanical stirrer, CTC resin (1.0-1.7meq/g,1 .0 equiv.) was mixed with a solution of a selected Fmoc-protected amino acid(1.1 equiv.) and DIPEA (6 equiv.) in a mixed solvent of DMF/DCM (1:10 v/v76.6 ml/mmol of CTC resin). The mixture was gently stirred at r.t. for 5 h. Anhydrousmethanol (16 equiv.) was added to cap any unreacted CTC resin. After being stirred atr.t. for another 30 mm, the solution was removed from the resin by vacuum filtration.The resin-bound product was washed with DMF (3x200 ml), DCM (3x200 ml), MeOH(3x200 ml), and DMF (3x200 ml). For Fmoc removal the resin was then treated with20% v/v piperidine in DMF (300 ml) at r.t. with gentle stirring for 30 mm. The resin wasthen filtered under vacuum, washed with DMF (3x200 ml), DCM (3x200 ml), MeOH (3x100 ml) and dried completely under vacuum to afford the CTC resin-bound amino acid which was used in solid phase synthesis directly without any further purification. The resin loading rate was estimated based on the weight increase compared to the non-loaded resin.; (1.0 equiv.) was loaded onto CTC resin using Method C. In a SPPS vessel equipped with overhead mechanical stirrer, the resin15 bound N6-allyloxycarbonyl-L-ornithine was mixed with a N-protected amino acid(1.3 equiv), HBTU (1.3 equiv.) and DMF (6-6.6 ml/mmol of loaded resin). The mixture was gently stirred at r.t. for about 2 h or until LC-MS indicated the completion of the reaction using Method A. The resin was filtered, washed with DMF (3x200 ml), DCM (3x200 ml) and MeOH (3x100 ml), then dried under vacuum. Alloxycarbonyl removalTo the SPPS vessel containing the resin-bound dipeptide (1.0 equiv) was added DCM(8-9 ml/mmol), phenyl silane (16 equiv.) and tetrakis(triphenylphosphine)palladium(0.12 equiv.) sequentially. The resulting mixture was gently stirred (about 50 rpm) at r.t.for 1 h under N2 The resin-bound product was filtered, washed with DCM (3x200 ml),DMF (3x200 ml) and MeOH (3x100 ml) and then dried under vacuum to afford the resin- bound dipeptide with a free ornithine 5-amino group.Further coupling of amino acidsTo the SPPS vessel containing the resin-bound dipeptide with a free ornithine 5-amino group was added a Fmoc-protected amino acid (1.5 equiv.), HBTU (1.5 equiv.), DMF (6-7 ml/mmol of loaded resin) and DIPEA (3 equiv.). The mixture was stirred gently at r.t. for 2 h or until LC-MS indicated completion of the reaction using Method A. The reaction solution was then removed from the SPPS vessel by vacuum filtration to affordthe resin-bound product, which was rinsed with DMF (3x200 ml), DCM (3x200 ml), MeOH (3x100 ml) and DMF (3x100 ml). For FMOC removal, the resin was subsequently treated with 20% v/v piperidine in DMF (8-9 ml/mmol of loaded resin) at r.t. with gentle stirring for 30 mm or until LC-MS indicated completion of the reactionusing Method A. The resin was vacuum filtered and rinsed with DMF (3x200 ml), DCM (3x200 ml) and MeOH (3x100 ml), then dried under vacuum to afford the resin-bound peptide with a free terminal amino group, which was used directly in the next amino acid coupling reaction without any further purification.The above coupling/de-Fmoc procedure was repeated three more times, each time using a different amino acid respectively to afford the CTC resin-bound linear hexapeptide sequence with a free terminal amino group.; The resin-bound pentapeptide or hexapeptide was treated with a solution of HFIPA inDCM (20% v/v7 12 ml/mmol of substrate) in an SPPS vessel with gentle stirring at r.t. for30 mm. The mixture was filtered and filtrate collected. The resin was treated withanother identical volume of 20% v/v HFIPA in DCM at r.t. upon gentle stirring for another 30 mm, and filtered again. The filtrates were combined and evaporated under vacuum to dryness to afford the linear pentapeptide or hexapeptide.; Following Method C, was loaded onto 2- chlorotrityl resin (CTC resin, 1.0 meq, 3.0 g, 3.0 mmol) and subsequently treated with piperidine in DMF (20% v/v) to afford the CTC resin-bound N6-allyloxycarbonyl-L- ornithine (3.0 mmol).The hexapeptide linear sequence was subsequently assembled by following theprocedures described in Method G using sequentially N-(2-(tert-butoxy)-2-oxoethyl)-N-(tert-butoxycarbonyl)-L-phenylalanine (1.71 g, 4.5 mmol, 1.5 equiv.), Na(((9Hfluoren9yl)methoxy)carbonyl)-N1?-((2,2,4,6, 7-pentamethyl-2, 3-dihydrobenzofuran-5-yl)sulfonyl)-L-arginine (2.92 g, 4.5 mmol, 1.5 equiv.),(tert-butoxycarbonyl)-L-tryptophan (1.92 g, 4.5 mmol, 1.5 equiv.), N-(9-fluorenylmethyloxycarbonyl)-3-(cis-4-hydroxycyclohexyl)-D-alanine (1.80 g, 4.5 mmol,1.5 equiv.) and (2S,4S)-i -(((9H-fluoren-9-yl)methoxy)carbonyl)-4-((tert-butoxycarbonyl)amino)pyrrolidine-2-carboxylic acid (2.03 g, 4.5 mmol, 1.5 equiv.) toafford the resin-bound linear hexapeptide. This resin-bound product was subject to thecleavage conditions described in Method I to afford the linear hexapeptide as an oil,4.41 g, 100%. LC-MS (... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping