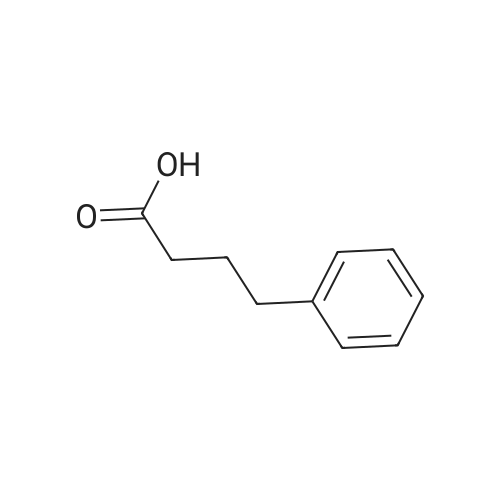
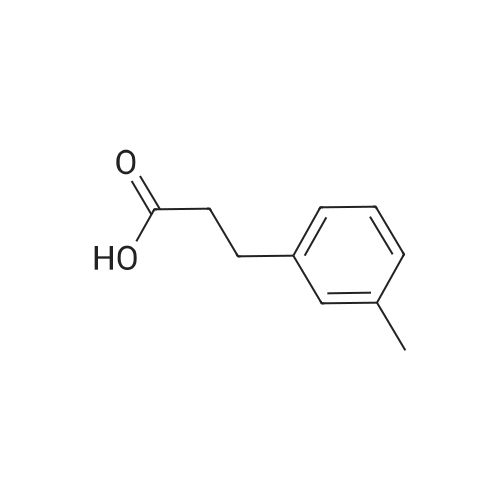
| 100% |
With hydrogen; potassium hydroxide; In water; at 20℃; under 760.051 Torr; for 1h;Green chemistry; |
General procedure: 1.0 mmol, 0.148 g), catalyst BPPd(0)Si (5 mol%, 0.0836 g), andKOH (1.0 equiv., 5 mL 0.2 M solution) were added to the reactionflask under hydrogen gas (1 atm). The reaction mixture was stirredat room temperature for 30 min followed by catalyst filtration andwashing with 10 mL of water and ethyl acetate. The pH was adjusted to 2e3 using 1 N HCl. The organic phase was collectedafter solvent extraction from ethyl acetate and dried over MgSO4and in vacuo. The product was purified by silica-gel column chromatographyand analyzed by 1H NMR spectroscopy. |
| 98% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; |
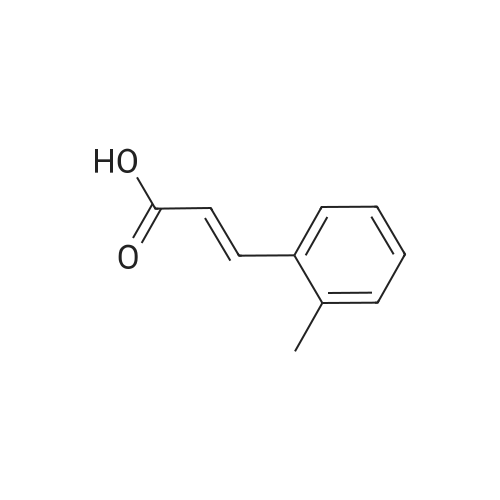
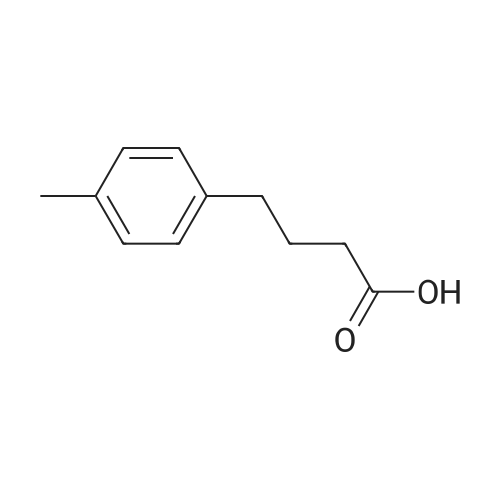
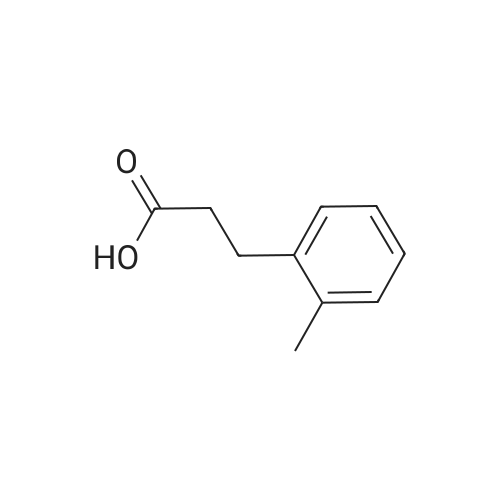
Briefly, into a 250-mL round-bottom flask, was placed a solution of 2- methylbenzaldehyde (8 g, 66.58 mmol, 1.00 equiv) in ethanol (80 mL), malonic acid (7.6 g, 73.03 mmol, 1.10 equiv), Pyridine (5 mL). The resulting solution was heated to reflux for 48 hr and allowed to cool to room temperature. The crystalline mass which formed was collect by filtration and washed with ethanol. This resulted in 6 g (55%) of (E -3-o- tolylacrylic acid as a white solid. Next, into a 250-mL round-bottom flask was placed a solution of (is)-3-o-tolylacrylic acid (12 g, 73.99 mmol, 1.00 equiv) in methanol (80 mL), Palladium carbon (2 g, 10%). Hydrogen was bubbled into the solution and the resulting solution was stirred overnight at room temperature. The solids were filtered out and the residue was concentrated under vacuum. This resulted in 12 g (98%) of 3-o-tolylpropanoic acid as colorless oil. Next, a solution of 3-o-tolylpropanoic acid (12 g, 73.08 mmol, 1.00 equiv) in TfOH (70 mL) was placed into a 250-mL round-bottom flask. The resulting solution was stirred overnight at room temperature. Then, ice-water was added and extracted with DCM. The combined organic phases were dried over anhydrous Na2S04. After filtration and concentration, the residue was applied onto a silica gel column with EA/PE=1/100 to 1/50. This resulted in 10.6 g (98%) of 4-methyl-2,3-dihydroinden-l-one as a white solid. Next, a solution of l-((2-(trimethylsilyl)ethoxy)methyl)-lH-imidazole (270 mg, 1.36 mmol, 1.00 equiv) in tetrahydrofuran (15 mL) was placed into a 100-mL 3- necked round-bottom flask. This was followed by the addition of n-BuLi (0.55 mL, 2.5M) with dropwise under N2 and stirred for 1 h at -70C. To this was added 4-methyl-2,3- dihydroinden-l-one (200 mg, 1.37 mmol, 1.00 equiv) in tetrahydrofuran (5 mL) dropwise. The reaction mixture was warmed to room temperature over a period of 1 h and the mixture was continued to stir overnight at rt. Then water was added and extracted with EA. The combined organic phases were dried over anhydrous a2S04. After filtration and concentration, the residue was purified by MPLC. This resulted in 250 mg (53%) of 4- methyl- 1 -(1 -((2-(trimethylsilyl)ethoxy)methyl)- 1 H-imidazol-2-yl)-2,3 -dihydro- 1 H-inden- l-ol as colorless oil. Finally, a solution of 4-methyl-l-(l-((2- (trimethylsilyl)ethoxy)methyl)- 1 H-imidazol-2-yl)-2,3 -dihydro- 1 H-inden- 1 -ol ( 100 mg, 0.29 mmol, 1.00 equiv) in HCOOH (10 mL), Palladium carbon (10 mg) was placed into a 100 mL round bottom flask. The resulting solution was heated to reflux for one overnight. The pH value of the solution was adjusted to 8 with aqueous sodium bicarbonate solution and extracted with EA. The combined organic phases were dried over anhydrous Na2S04. After filtration and concentration, the residue was purified by MPLC. This resulted in 40 mg (67%) of 2-(4-methyl-2,3-dihydro-lH-inden-l-yl)-lH-imidazole as a white solid. LCMS(m/e) 199 (M+H); XH NMR (300 MHz, CDC13) delta ppm 6.96-7.18 (m, 3H), 6.93 (s, 2H), 4.59 (t, J=8.1 Hz, 1H), 2.80-3.00 (m, 2H), 2.50-2.62 (m, 1H), 2.29 (s, 3H), 2.45-2.29 (s, 1H). |
| 98% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; |
Briefly, into a 250-mL round-bottom flask, was placed a solution of 2-methylbenzaldehyde (8 g, 66.58 mmol, 1.00 equiv) in ethanol (80 mL), malonic acid (7.6 g, 73.03 mmol, 1.10 equiv), Pyridine (5 mL). The resulting solution was heated to reflux for 48 hr and allowed to cool to room temperature. The crystalline mass which formed was collect by filtration and washed with ethanol. This resulted in 6 g (55%) of (E)-3-o-tolylacrylic acid as a white solid. Next, into a 250-mL round-bottom flask was placed a solution of (E)-3-o-tolylacrylic acid (12 g, 73.99 mmol, 1.00 equiv) in methanol (80 mL), Palladium carbon (2 g, 10%). Hydrogen was bubbled into the solution and the resulting solution was stirred overnight at room temperature. The solids were filtered out and the residue was concentrated under vacuum. This resulted in 12 g (98%) of 3-o-tolylpropanoic acid as colorless oil. Next, a solution of 3-o-tolylpropanoic acid (12 g, 73.08 mmol, 1.00 equiv) in TfOH (70 mL) was placed into a 250-mL round-bottom flask. The resulting solution was stirred overnight at room temperature. Then, ice-water was added and extracted with DCM. The combined organic phases were dried over anhydrous Na2SO4. After filtration and concentration, the residue was applied onto a silica gel column with EA/PE=1/100 to 1/50. This resulted in 10.6 g (98%) of 4-methyl-2,3-dihydroinden-1-one as a white solid. Next, a solution of 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-imidazole (270 mg, 1.36 mmol, 1.00 equiv) in tetrahydrofuran (15 mL) was placed into a 100-mL 3-necked round-bottom flask. This was followed by the addition of n-BuLi (0.55 mL, 2.5M) with dropwise under N2 and stirred for 1 h at -70 C. To this was added 4-methyl-2,3-dihydroinden-1-one (200 mg, 1.37 mmol, 1.00 equiv) in tetrahydrofuran (5 mL) dropwise. The reaction mixture was warmed to room temperature over a period of 1 h and the mixture was continued to stir overnight at rt. Then water was added and extracted with EA. The combined organic phases were dried over anhydrous Na2SO4. After filtration and concentration, the residue was purified by MPLC. This resulted in 250 mg (53%) of 4-methyl-1-(1-((2-(trimethylsilyl)ethoxy)methyl)-1H-imidazol-2-yl)-2,3-dihydro-1H-inden-1-ol as colorless oil. Finally, a solution of 4-methyl-1-(1-((2-(trimethylsilyl)ethoxy)methyl)-1H-imidazol-2-yl)-2,3-dihydro-1H-inden-1-ol (100 mg, 0.29 mmol, 1.00 equiv) in HCOOH (10 mL), Palladium carbon (10 mg) was placed into a 100 mL round bottom flask. The resulting solution was heated to reflux for one overnight. The pH value of the solution was adjusted to 8 with aqueous sodium bicarbonate solution and extracted with EA. The combined organic phases were dried over anhydrous Na2SO4. After filtration and concentration, the residue was purified by MPLC. This resulted in 40 mg (67%) of 2-(4-methyl-2,3-dihydro-1H-inden-1-yl)-1H-imidazole as a white solid. LCMS (m/e) 199 (M+H); 1H NMR (300 MHz, CDC3) delta ppm 6.96-7.18 (m, 3H), 6.93 (s, 2H), 4.59 (t, J=8.1 Hz, 1H), 2.80-3.00 (m, 2H), 2.50-2.62 (m, 1H), 2.29 (s, 3H), 2.45-2.29 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping