|
|
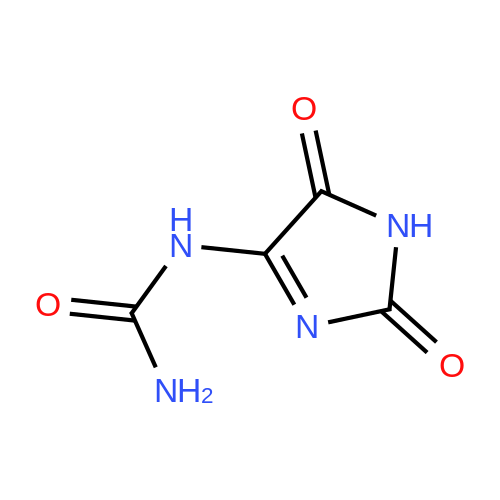
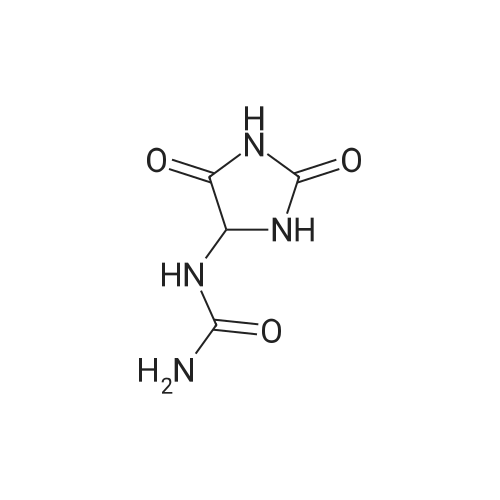
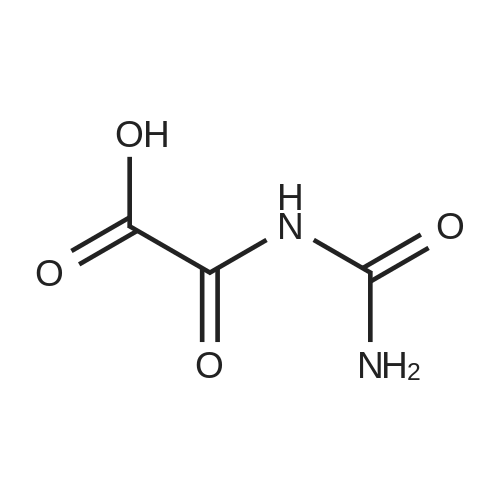
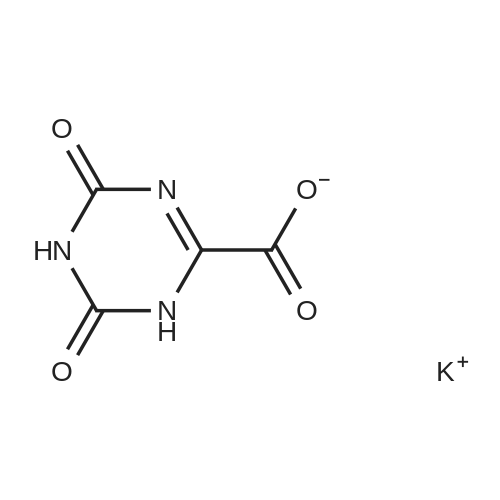
EXAMPLE 1 In a flask with a volume of 500 ml, 271 g of 16.6% aqueous potassium hydroxide solution was added. Then, 15.8 g of allantoin and 0.79 g of potassium iodide were added thereto and dissolved, while keeping the internal temperature in the range of 0 to 10 C. Thereafter, 32.0 g of bromine was added dropwise thereto at such a rate as to keep the internal temperature at 2 to 5 C. for approximately 2.5 hours. After completion of the addition of bromine, the internal temperature was raised to 20 C., followed by stirring for approximately 22 hours. Then, the reaction solution was neutralized with 21.5 g of acetic acid to a pH of approximately 6 to precipitate crystals. Subsequently, the reaction solution was cooled to approximately 5 C., followed by stirring for 2 hours. Thereafter, the crystals were filtered off, and the obtained crystals were washed with 66 ml of cold water, and 22 ml of cold acetone, successively. Then, the crystals were dried to obtain 13.7 g of potassium oxonate. The yield of the resulting potassium oxonate from allantoin was 70%. The physical properties of the obtained potassium oxonate are shown below. IR (KBr) nu (cm-1): 3170, 1750, 1720, 1655, 1622, 1490, 1395, 780 13 C-NMR (100 MHz, heavy water) delta (ppm): 160.06, 164.35, 158.63 |
|
|
EXAMPLE 2 Into a flask with a volume of 500 ml, 15.8 g of allantoin, 118.5 ml of water, 13.9 g of 85% potassium hydroxide and 0.79 g of potassium iodide were added, followed by mixing and dissolution while keeping the inner temperature at 0 to 5 C. Then, 181.1 g of a commercially available aqueous solution of potassium hypochlorite (chlorine content in the solution: 5 to 7%) was added dropwise thereto over approximately 2.5 hours while keeping the internal temperature at 2 to 5 C. After completion of the addition of the aqueous solution of potassium hypochlorite, the internal temperature was raised to 25 C., followed by stirring for approximately 18.5 hours. Then, the reaction solution was neutralized with 9.17 g of acetic acid to a pH of approximately 6 to precipitate crystals. Subsequently, the reaction solution was cooled to approximately 5 C., followed by stirring for 2 hours. Thereafter, the crystals were filtered off, and the obtained crystals were washed with 66 ml of cold water, and 22 ml of cold acetone, successively, and dried to obtain 8.6 g of potassium oxonate. The yield of the resulting potassium oxonate from allantoin was 44%. Further, the IR of the resulting potassium oxonate was found to be the same as in the example 1. |
|
|
EXAMPLE 3 Into a flask with a volume of 500 ml, 15.8 g of allantoin, 118.5 ml of water, 13.9 g of 85% potassium hydroxide and 0.79 g of potassium iodide were added, followed by mixing and dissolution, while keeping the internal temperature at 0 to 5 C. Then, 132.9 g of a commercially available aqueous solution of sodium hypochlorite (chlorine content in the solution: approximately 5%) was added dropwise thereto over approximately 2.5 hours while keeping the internal temperature at 2 to 5 C. After completion of the addition of the aqueous solution of sodium hypochlorite, the internal temperature was raised to 25 C., followed by stirring for approximately 23 hours. Then, the reaction solution was neutralized with 11.28 g of acetic acid to a pH of approximately 6 to precipitate crystals. Subsequently, the reaction solution was cooled to approximately 5 C., followed by stirring for 1 hour. Thereafter, the crystals were filtered off, and the obtained crystals were washed with 66 ml of cold water, and 22 ml of cold acetone, successively, and dried to obtain 7.8 g of potassium oxonate. The yield of the resulting potassium oxonate from allantoin was 40%. The IR of the resulting potassium oxonate was found to be the same as in the example |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping