| 42% |
|
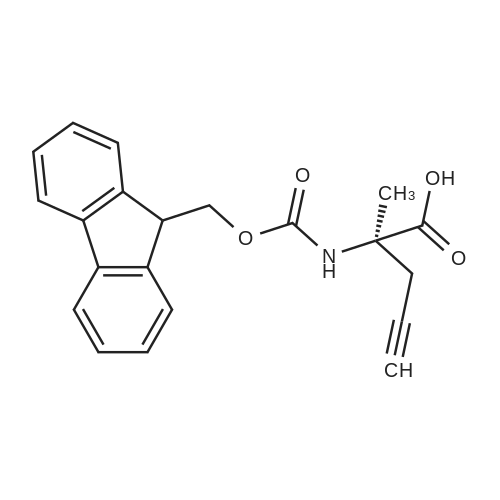
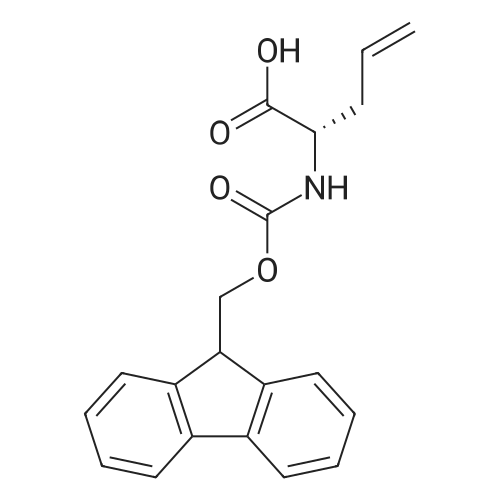
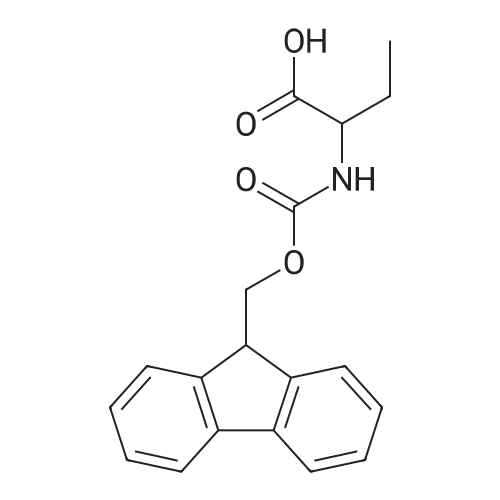
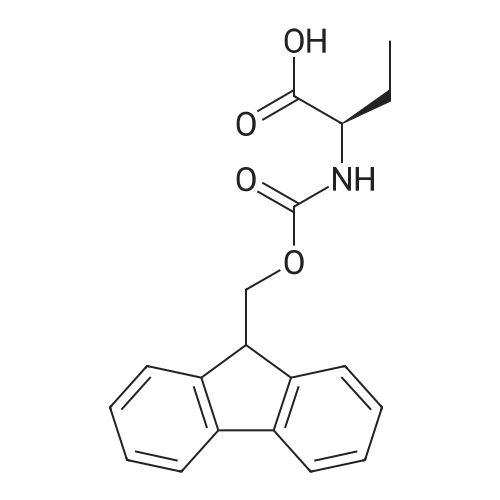
General procedure: Peptide synthesis was conducted manually in disposable Torviq polypropylene syringes fitted with a Teflon sinter. All reactant equivalents are based on the resin loading level for a given amount of resin. Loading of Amino Acid onto Rink Amide AM Resin and Capping. Rink Amide AM resin at a loading level of 0.41 mmol g-1 was placed in a sinter-fitted syringe and allowed to swell in anhydrous CH2Cl2 for 1 h. After removal of CH2Cl2, the resin was treated with a solution of 20percent v/v piperidine in DMF (35 mL 10 min) and the resin was then drained and rinsed with DMF (3 x 5 mL), CH2Cl2 (3 x 5 mL), and DMF (3 x 5 mL). Afterwards, a solution of Fmoc-protected amino acid (1.5 equiv.), N,N-diisopropylethylamine (DIPEA) (3.0 equiv.), and HATU (2.5 equiv.) in DMF (0.06 M) was added to the reaction syringe. After agitation at room temperature for 2 h, the resin was filtered off and washed with DMF (3 x 5 mL) and CH2Cl2 (3 x 5 mL). Capping was accomplished by treatment of the resin with 20percent v/v acetic anhydride in pyridine (35 min) and subsequent washing with DMF (3 x 6 mL), CH2Cl2 (3 x 6 mL),and DMF (3 x 6 mL). Click Reaction on Resin. The resin-bound peptide was transferred to a 10 mL flask containing CuSO4*5H2O and sodium ascorbate, a solution of azide (2 equiv. or 4 equiv. relative to resin capacity) in DMF (0.05 M) was then added. The suspension was allowed to stirat 25 °C for 16 or 40 h and filtered through a 10 mL syringe tube fitted with a frit. The resin was then rinsed successively with DMF (3 x 5 mL), DDTC/DMF solution (1 g DDTC in 100 mL DMF, 15 x 6 mL), CH2Cl2 (3 x 5 mL), and DMF (3 x 5 mL). Fmoc Deprotection. Fmoc deprotection was carried out by suspending the resin in 20percent v/v piperidine/DMF (3 x 5 mL x 10 min) and agitating the syringe at room temperature for 3 x 10 min. The suspension wasthen filtered and the resin was washed with DMF (3 x 5 mL), CH2Cl2 (3 x 5 mL), and DMF (3 x 5 mL). SPPS Peptide Coupling. A solution of Fmoc-protected amino acid (1.5 equiv.), DIPEA (3.0 equiv.), and HATU (2.5 equiv.) in DMF (0.06 M) was added to the reaction syringe containing the resin. The resulting suspension was agitated at room temperature for 2 h and the resin was then rinsed with DMF (3 x 5 mL), CH2Cl2 (3 x 5 mL), and DMF (3 x 5 mL). Acetylation. After Fmoc deprotection, the resin was treated with 20percent v/v acetic anhydride/pyridine (3 x 5 mL x 5 min), followed by washing with DMF (3 x5 mL), CH2Cl2 (3 x 5 mL), and DMF (3 x 5 mL). Cleavage of Peptides from the Resin. After acetylation, the resin was washed with DMF (3 x 5 mL) and CH2Cl2 (3 x 5 mL). The resin was then treated with a solution of TFA/H2O/TIS (95 : 2.5 : 2.5 v/v, 21 h) and washed with CH2Cl2 (2 x 2 mL). All solutions were combined and evaporated to give the desired crude product. Tripeptide 6. Tripeptide 6 was prepared following the procedure described above for 5 in 42percent yield; [a]D20 +54.2 (c 0.20 MeOH). nmax/cm-1 3345 (br), 1756, 1679, 1613, 1601, 1472, 1375, 1225. dH (500 MHz, CD3OD) 8.38 (d, J 4.5, 4H), 7.85 (s, 1H), 7.68 (m, 6H), 7.24 (m, 8H), 7.07 (s, 1H), 6.71 (s, 1H), 5.74 (s, 2H), 5.63 (s, 1H), 4.66 (m, 1H), 4.52 (m, 6H), 3.80 (m, 8H), 3.16 (m, 3H), 3.06 (m, 2H), 2.98 (m, 5H), 1.89 (s, 3H). dC (125 MHz, CD3OD) 175.6, 173.5, 173.4, 172.9, 163.3, 159.9, 152.5, 151.6, 150.2, 149.4, 144.9, 144.8, 144.7, 144.2, 138.7, 125.6, 125.0, 124.8, 123.9, 110.9, 110.6, 109.3, 104.1, 60.6, 54.9, 54.8, 54.4, 51.1, 28.6, 28.5, 28.0, 22.6. HRMS (ESI) 1136.4668; calcd. for C55H59N19O8Na [M+Na]+ 1136.4686. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping