| 68% |
|
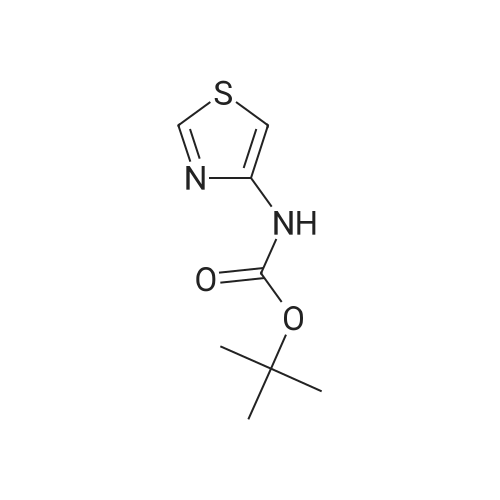
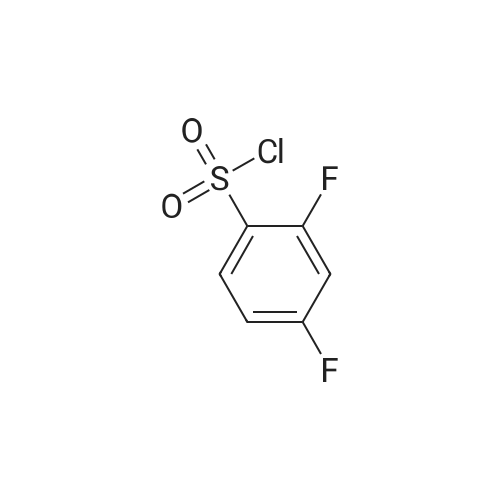
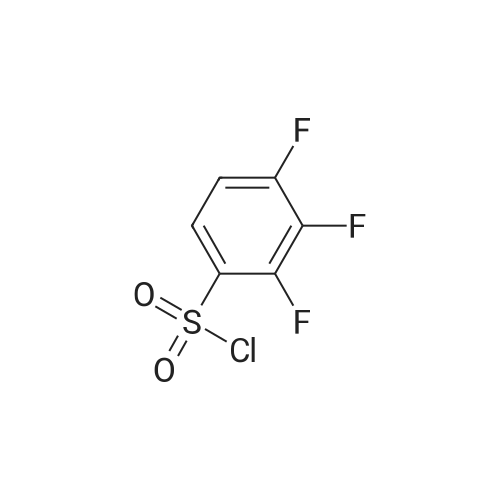
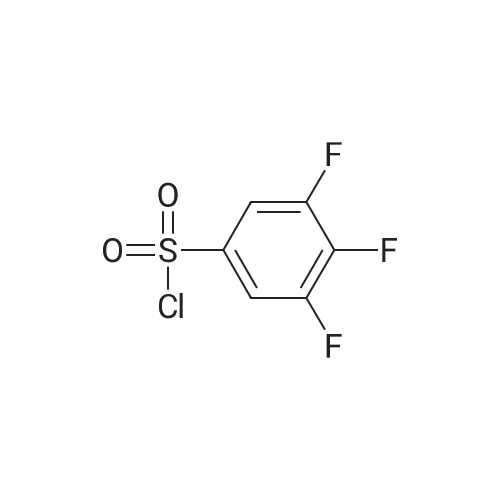
EXAMPLE 293 Synthesis of (S)-4-((1-benzylpyrrolidin-3-yl)(methyl)amino)-2,5-difluoro-//-(thiazol-4- yl)benzenesulfonamide 2,2,2-trifluoroacetate Step 1. Preparation of te/f-butyl thiazol-4-yl((2,4,5-trifluorophenyl)sulfonyl)carbamate To a solution of terf-butyl thiazol-4-ylcarbamate (1.47 g, 7.35 mmol) in anhydrous tetrahydrofuran (10 mL) was added a 1 M solution of lithium bis(trimethylsilyl)amide in tetrahydrofuran (10.3 mL, 10.3 mmol) at -78 C. The reaction mixture was allowed to warm to ambient temperature and stirred for 1 h. The reaction mixture was then cooled to -78 C, and a solution of 2,4,5- trifluorobenzenesulfonyl chloride (1.22 mL, 8.82 mmol) in anhydrous tetrahydrofuran (10 mL) was added to it. The reaction mixture was allowed to warm to ambient temperature and stirred for 3 h. The mixture was diluted with ethyl acetate (50 mL), washed with saturated ammonium chloride (2 chi 30 mL), brine (2 chi 30 mL), dried over anhydrous sodium sulfate, and filtered. Concentration of the filtrate in vacuo and purification of the residue by column chromatography, eluting with a gradient of 0 to 50% of ethyl acetate in hexanes, provided the title compound as a colorless solid (2.0 g, 68% yield): MS (ES+) m/z 395.0 (M + 1). |
| 64% |
|
To a solution of terf-butyl 1 ,3-thiazol-4-ylcarbamate (Preparation 3, 28.94 g, 145 mmol) in anhydrous tetrahydrofuran (600 mL) at -70 C, under an atmosphere of nitrogen was added lithium 1 , 1 ,1 ,3,3,3-hexamethyldisilazan-2-ide (1 M in tetrahydrofuran , 145 mL, 1 45 mmol ) d rop-wise. The reaction mixture was allowed to warm to ambient temperature and stirred for 1 hour before cooling to -70 C once again. A solution of 2, 4, 5-trifluro benzenesulfonyl chloride (40 g, 173 mmol) in tetrahydrofuran (80 mL) was added drop-wise and then th e reaction m ixtu re was slowly warmed to am b ient temperature and stirred for 2 hours. The reaction mixture was quenched with saturated aqueous ammonium chloride solution and extracted with ethyl acetate. The organic layer was washed with water and saturated aqueous sodium chloride solution before concentrating in vacuo. The crude residue was purified by silica gel column chromatography (0% to 15% ethyl acetate in hexanes gradient elution) to afford the title compound as white solid (37 g, 64%).1 H NMR (400 MHz, CDCI3): delta 1 .35 (s, 9H), 7.07-7.1 3 (m, 1 H), 7.52 (s, 1 H), 8.00-8.06 (m, 1 H), 8.78 (s, 1 H).LCMS Rt= 3.46 minutes. MS m/z 395 [MH]+ |
| 62% |
|
A solution of ferf-butyl thiazol-4-ylcarbamate (3.46 g, 17.3 mmol) in tetrahydrofuran (150 mL) at -78 C was treated with lithium 6/'s(trimethylsilyl)amide (1.0 M solution in tetrahydrofuran, 20.8 mL, 20.8 mmol). The resulting mixture was stirred at -78 C for 0.5 h, allowed to warm to ambient temperature and stirred for a further 0.5 h. The reaction mixture was cooled to -78 C and treated with a solution of 2,4,5- trifluorobenzene-1-sulfonyl chloride (3.99 g, 17.3 mmol) in tetrahydrofuran (30 mL). The resulting mixture was stirred at -78 C for 4 h, allowed to warm to ambient temperature and stirred for a further 16 h. The reaction mixture was diluted with ethyl acetate (300 mL) and washed with saturated aqueous ammonium chloride (2 x 150 mL) and brine (2 x 150 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The residue was purified by column chromatography eluting with a gradient of ethyl acetate in hexanes to afford terf-butyl thiazol-4-yl((2,4,5- trifluorophenyl)sulfonyl)carbamate as a beige solid in 62% yield (4.23 g): 1H NMR (300 MHz, CDCI3) 6 8.79-8.75 (m, 1 H), 8.06-7.96 (m, 1 H), 7.53-7.48 (m, 1H), 7.15-7.04 (m, 1 H), 1.34 (s, 9H); MS (ES+) m/z 394.7 (M + 1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping