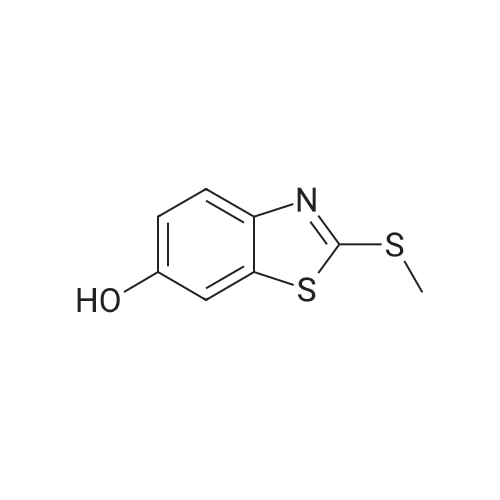
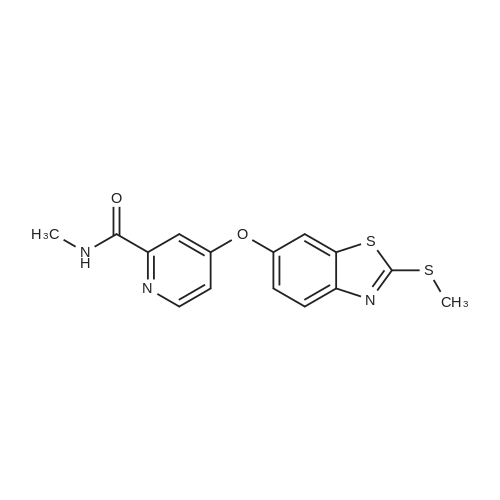
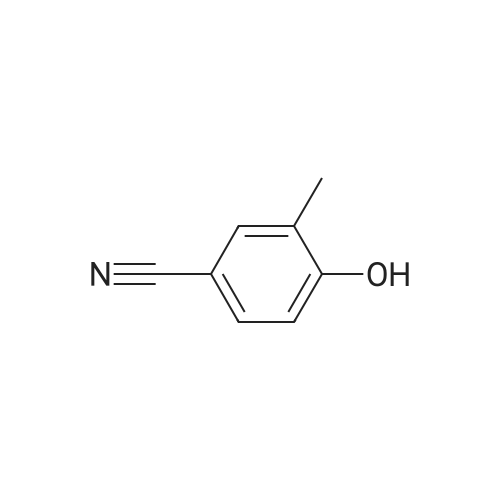
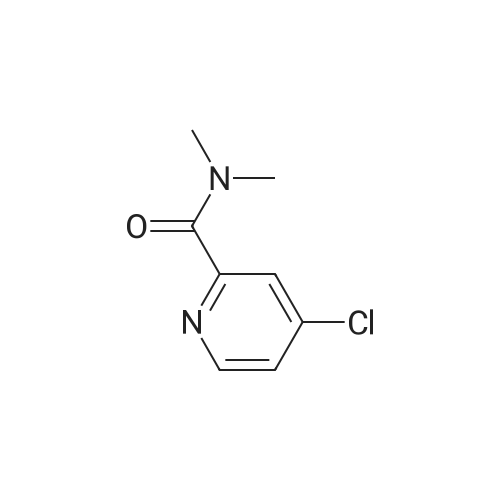
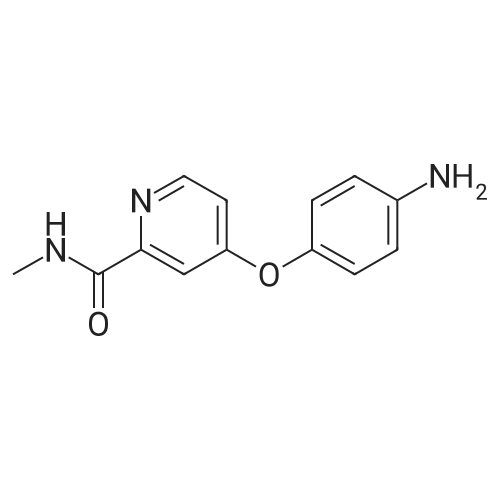
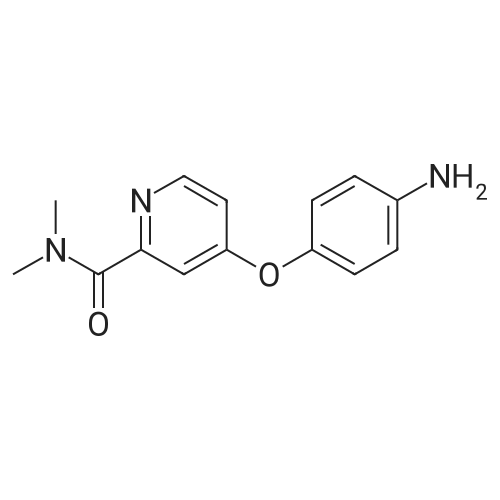
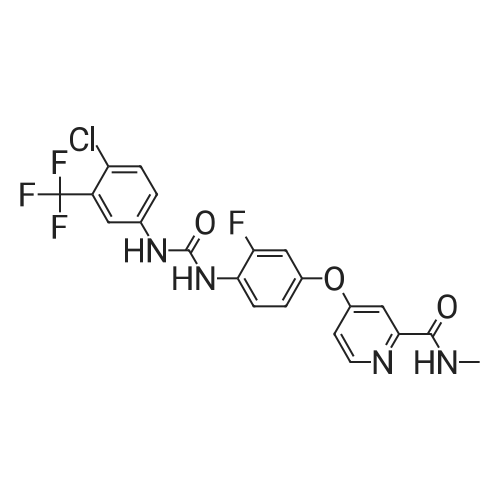
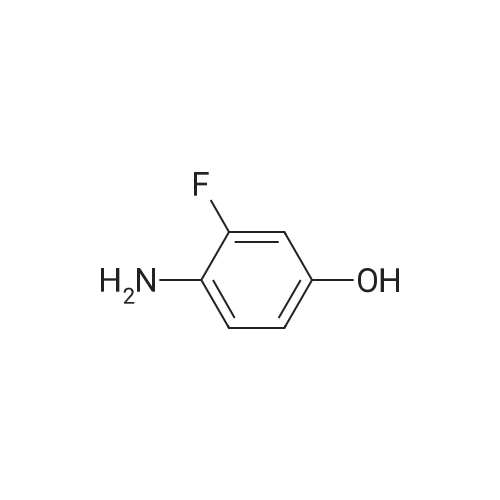
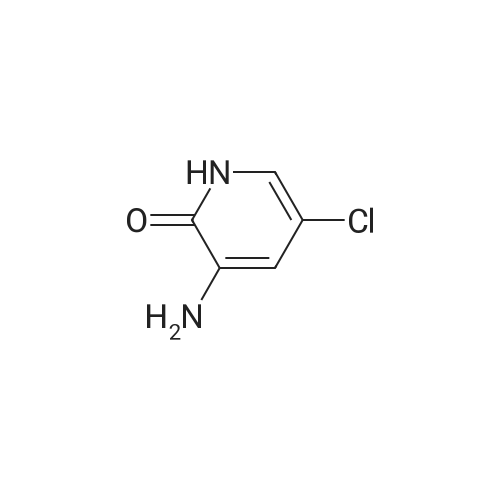
| 136 mg |
|
Example 2.2.: Synthesis of 4-{2- ethyl-4-[3-(1-methyl-2-oxo-5- trifluoromethyl-1,2-dihydro-pyridin-3-yl)-ureidomethyl]-phenoxy}- pyridine-2-carboxylic acid methyl amide 1) Synthesis of 4-(4-Cvano-2-methyl-phenoxy)-pyridine-2-carboxylic acid methylamide A solution of 4-Hydroxy-3-methyl-benzonitrile (0,100 g; 0,717 mmol) in dry DMF (3 ml_) was treated with potassium-terf-butylat (0,088 g; 0,788 mmol). The reaction mixture was stirred at RT for 2 h and 4-Chloro-pyridine-2- carboxylic acid methylamide (0,130 g; 0,717 mmol) and potassium carbonat (0,020 ml; 0,358 mmol) were added. The resulting suspension was then heated to 130C for 4 days. For purification the reaction mixture was allowed to cool down to RT and it was washed with 1 N NaOH-solution (5 ml_) and water (5 ml_). The solid, that precipitated while washing, was filtrated and added into the organic layer. The aqueous phase was extracted with DCM (2 x 15 mL) and the combined organic layers were evaporated to dryness. The resulting solid was dissolved in DCM (20 mL), dried with Na2S04 and concentrated to afford the crude product. The product was purified with flash column chromatography (Combi Flash RF, Si-60, 24 g-column, gradient PE/EE 95:5 to 50:50 in 12 min then for 7 min isocratic 50:50, flow 35 ml/min, UV 254 nM) resulting in 4-(4-Cyano-2-methyl-phenoxy)-pyridine-2-carboxylic acid methylamide (136,000 mg; 0,443 mmol) as yellow solid. 2) Synthesis of 4-(4-Aminomethyl-2-methyl-phenoxy)-pyridine-2-carboxylic acid methylamide A solution of 4-(4-Cyano-2-methyl-phenoxy)-pyridine-2-carboxylic acid methylamide (0,690 g; 0,836 mmol) in methanol (5 mL) and NH3 in methanol (20%, 5 mL) was treated with nickel sponge (0.5 g Johnson Matthey, A- 7000) and purged with H2. The reaction mixture was stirred at RT for 17.5 h with a pressure of five bar. The catalyst was filtrated off and the solvent was evaporated. The crude product was then purified by flash column chromatography (Flashmaster, UV 240 nM, 70g silica gel column, flow 20 ml/min, DCM/MeOH 9:1) yielding 4-(4-Aminomethyl-2-methyl-phenoxy)- pyridine-2-carboxylic acid methylamide (0,182 g; 0,584 mmol) as yellow resin. 3) Synthesis of 1-Methyl-3-nitro-5-trifluoromethyl-1 H-pyridin-2-one To a solution of 3-Nitro-5-(trifluoromethyl)pyridin-2-ol (60,0 g; 288,33 mmol) in DMF (500 ml) was added potassium carbonat (120,0 g; 864,99 mmol) and iodomethan (19,7 ml; 317,16 mmol). The resulting suspension was stirred for about 16 h at 80C. The reaction mixture was diluted with EtOAc and extracted 3x with water, dried over Na2SO4, filtrated and the solution evaporated to dryness. The residue was treated with THF/ petroleum ether (PE). The precipitated product was filtered off, rinsed with PE and dried in vacuo to yield a brown solid. 4) Synthesis of 3-Amino-1-methyl-5-trifluoromethyl-1H-pyridin-2-one To a solution of 1-Methyl-3-nitro-5-trifluoromethyl-1 H-pyridin-2-one (9.40 g, 42.32 mmol) in THF (100 ml) and MeOH (10 ml) was added 5% Pd/C (54% H20, 2 g). The reaction was stirred under an atmosphere of hydrogen at room temperature. After 16h additional Pd/C (4g) were added and stirring was continued under hydrogen (1 atm) for 23 hours. The solids were removed via filtration and the filtrate reduced in vacuo to yield 3-Amino-1- methyl-5-trifluoromethyl-1H-pyridin-2-one (7.9 g, 41.1 mmol). 5) Synthesis of 4-f2-Methyl-4-r3-(1-methyl-2-oxo-5-trifluoromethyl-1 ,2- dihvdro-pyridin-3-yl)-ureidomethyll-phenoxy -pyridine-2-carboxylic acid methyl amide 3-Amino-1-methyl-5-trifluoromethyl-1H-pyridin-2-one (1,64 g, 8,55 mmol) was dissolved in DCM (50 ml), 4-nitro-phenyl-chloro-formiate (1 ,90 g, 9,437 mmol) and pyridine (0,76 ml) were added. The mixture was stirred for 2 h at room temperature. Then were added 4-(4-Aminomethyl-2-methyl-phenoxy)- pyridine-2-carboxylic acid methylamide (2,32 mg, 8,55 mmol) and N-ethyl- diisopropyl-amine (2,91 ml, 17,10 mmol). The mixture was stirred for about 16 h at room temperature. To the mixture was added DCM. The organic layer was washed once with 1 N NaOH and twice with water, it was dried over Na2S04, filtrated and the solvent removed in vacuo. The residual mixture was taken up with MTBE, the resulting white precipitate was filtered off and dried in vacuo. 4-{2-Methyl-4-[3-(1-methyl-2-oxo-5-trifluoromethyl-1 ,2-dihydro-pyhdin-3-yl)- ureidomethyl]-phenoxy}-pyridine-2-carboxylic acid methylamide was obtained as white solid (HPLC/MS: Rt=2,184 min, M+H 490.2). 1H NMR (500 MHz, DMSO-d6) ppm = 8.74 (q, J=4.6, 1H), 8.65 (s, 1 H), 8.50 (d, J=5.6, 1 H), 8.22 (d, J=2.5, 1 H), 7.96 - 7.92 (m, 1 H), 7.76 (t, J=5.9, 1 H), 7.34 - 7.31 (m, 1H), 7.29 (d, J=2.6, 1 H), 7.25 (dd, J=8.2, 2.2, 1 H), 7.15 - 7.11 (m, 1H), 7.10 (dd, J=5.6, 2.6, 1H), 4.33 (d, J=5.8, 2H), 3.57 (s, 3H), 2.79 (d, J=4.9, 3H), 2.10 (s, 3H). Method Info: HPLC/MS A: H20 + 0,05% HCOOH | B: MeCN + 0,04% HCOOH T: 30 C I Flow: 2 ml/min | Column: Chromolith RP-18e 50-4,6 mm | MS: 85- 800 amu 1% -> 00% B: 0 -> 2,8 min | 100% B: 2,8 -> 3,3 min |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping