| 100% |
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 80℃; for 2h;Inert atmosphere; |
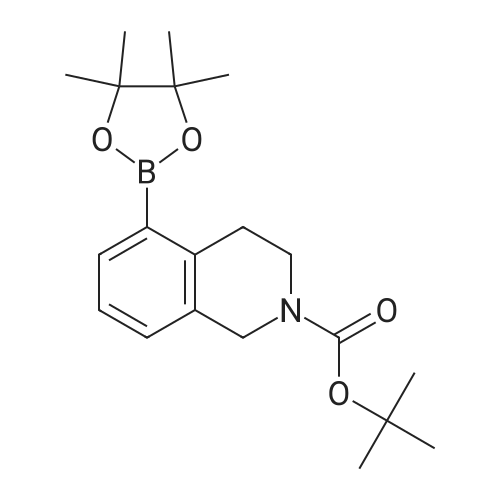
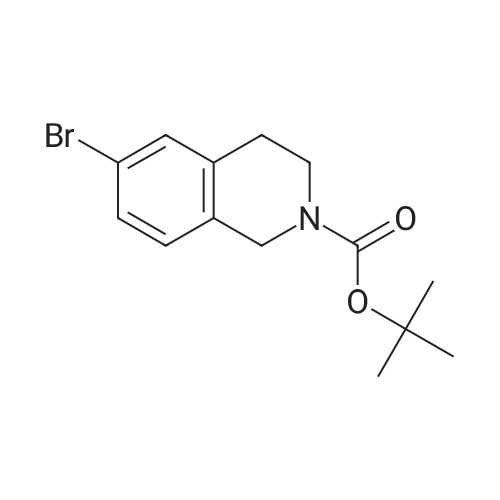
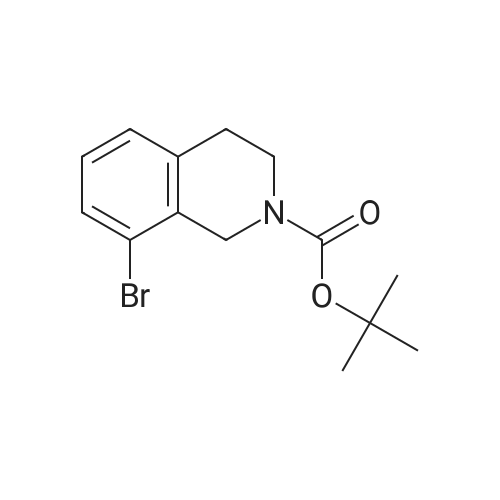
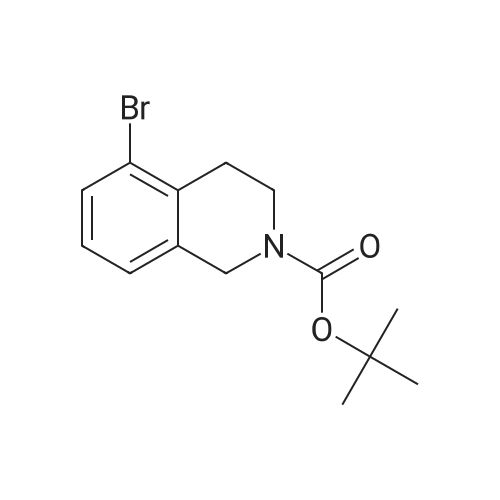
1 ,1-Dimethylethyl 5-bromo-3,4-dihydro-2(1 H)-isoquinolinecarboxylate (Preparation 53) (2.7g, 8.7 mmol), potassium acetate (2.55g, 26 mmol), [1 ,1'- bis(diphenylphosphino)ferrocene]dichloropalladium(ll) (0.63g, 0.87 mmol) and 4.4.4'.4'.5.5.5'.5'-octamethyl-2.2'-bi-1 .3,2-dioxaborolane (4.39g, 17 mmol) were dissolved in 1 ,4-dioxane (40ml), stirred at 800C under nitrogen for 2h and allowed to cool. Water (30ml) was added and the mixture was extracted with ethyl acetate (3x 20ml).The combined organic phases were concentrated in vacuo. Purification of the residue by flash chromatography (ethyl acetate in cyclohexane 15%) gave 1 ,1- dimethylethyl 5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)-3,4-dihydro-2(1 H)- isoquinolinecarboxylate as a clear gel (3.25g, 105%). LCMS (Method formate): Retention time 1.51 min, MH+ = 360 |
| 89% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In N,N-dimethyl-formamide; at 90℃;Inert atmosphere; |
A mixture of tert-butyl 5-bromo-3,4-dihydroisoquinoline-2(lH)-carboxylate (0.500 g, 1.60 mmol) and 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (0.488 g, 1.92 mmol) in DMF (8 mL) was subjected to 3 evacuate-fill cycles with nitrogen. Potassium acetate (0.472 g, 4.80 mmol) and PdCi2(dppf) DCM complex (0.117 g, 0.160 mmol) were added, the mixture was subjected to 2 more evacuate-fill cycles with nitrogen, and heated 90 C overnight under a nitrogen atmosphere. The mixture was cooled to room temperature, diluted with EtOAc, washed sequentially with water, 10% aqueous LiCl and saturated brine, dried and concentrated. The residue was subjected to column chromatography on silica gel, eluting with EtOAc-hexanes, to provide tert-butyl 5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-3,4-dihydroisoquinoline-2(17i)- carboxylate as a white solid (0.514 g, 89% yield). Mass spectrum m/z 360 (M+H)+. |
| 57% |
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 80℃; for 2h;Inert atmosphere; |
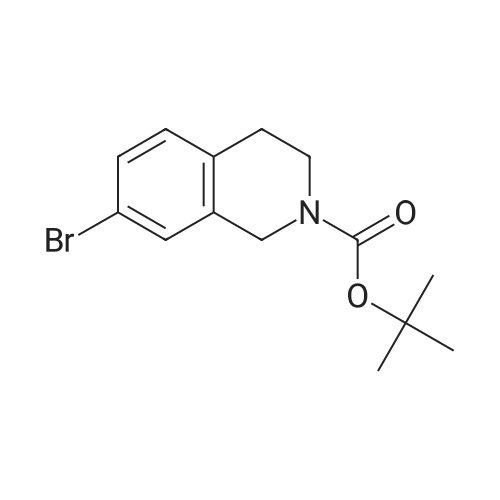
1 ,1-Dimethylethyl 5-bromo-3,4-dihydro-2(1H)-isoquinolinecarboxylate (Preparation 27) (0.281 g, 0.899 mmol), potassium acetate (0.265 g, 2.70 mmol), [1,f- bis(diphenylphosphino)ferrocene]dichloropalladium(ll) (0.066 g, 0.090 mmol) and 4i4i4li4Ii5,5,5',5'-octamethyl-2,2'-bi-1I3,2-dioxaborolane (0.274 g, 1.079 mmol) were dissolved in 1 ,4-dioxane (5 ml) and the resulting mixture was stirred at 800C under nitrogen for 2 hours then cooled to room temperature and diluted with water (15 ml). The aqueous phase was extracted with AcOEt (3 x 10 ml). The combined organic phases were dried over MgSO4 and concentrated in vacuo. Purification of the residue by flash chromatography on silica gel (c-Hexane/AcOEt: 15%) gave 1,1- dimethylethyl 5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)-3,4-dihydro-2(1 H)- isoquinolinecarboxylate 158 mg, 57%) as a light yellow oil. LCMS: retention time 1.54 min; no mass ion detected. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping