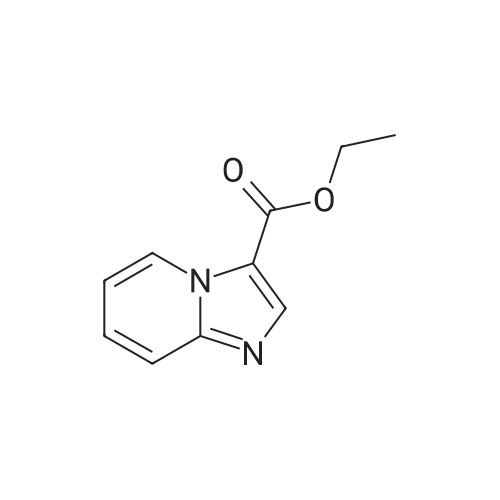
|
With lithium chloride;tetrakis(triphenylphosphine) palladium(0); In 1,4-dioxane; at 90℃; for 3.5h;Inert atmosphere; |
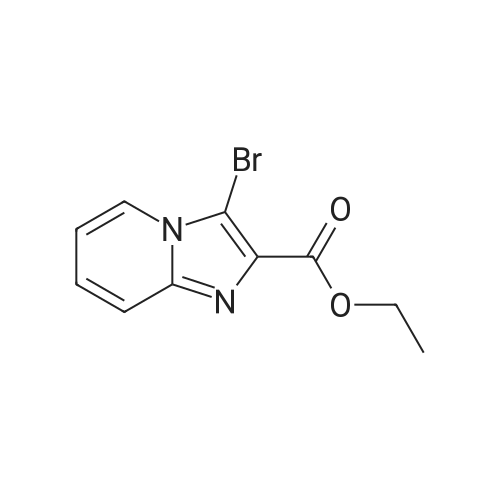
Intermediate 12: 6-(Oxazol-2-yl)imidazo[1,2-α]pyridine-2-carboxylic acid 12.1: Ethyl 6-(oxazol-2-yl)imidazo[1,2-α]pyridine-2-carboxylate 1 g of ethyl 6-iodoimidazo[1,2-α]pyridine-2-carboxylate, 350 mg of tetrakis(triphenyl-phosphine)palladium(0) and 360 mg of lithium chloride are degassed under vacuum and then suspended, under argon, in 15 ml of degassed dioxane. After addition of 5 g of 2-[tri(n-butyl)stannyl]oxazole, the reaction mixture is heated at 90 C. for 3.5 hours, then cooled and diluted and stirred with a mixture of 100 ml of 1M aqueous potassium fluoride solution and 200 ml of ethyl acetate. The aqueous phase is extracted with 200 ml of ethyl acetate and the combined organic phases are washed with aqueous sodium chloride solution and dried over sodium sulphate, filtered and concentrated to dryness under reduced pressure. The residue is chromatographed on silica, elution being carried out with a gradient of ethyl acetate and hexane (from 80/20 to 100/0). The fractions comprising the expected product are combined and concentrated to dryness under reduced pressure to give 530 mg of ethyl 6-(oxazol-2-yl)imidazo[1,2-α]pyridine-2-carboxylate in the form of a yellow powder.1H NMR spectrum (d6-DMSO, δ in ppm): 9.30 (d, J=0.8, 1H), 8.68 (s, 1H), 8.30 (s, 1H), 7.85 (dd, J=1.7, 9.5, 1H), 7.79 (d, J=9.5, 1H), 7.44 (d, J=0.6, 1H), 4.33 (q, J=7.0, 2H), 1.33 (t, J=7.1, 3H).Mass spectrum (APCI): m/z=258 [M+H]+. |
|
With lithium chloride;tetrakis(triphenylphosphine) palladium(0); In 1,4-dioxane; at 90℃; for 3.5h;Inert atmosphere; |
22.1: Ethyl 6-(oxazol-2-yl)imidazo[1,2-a]pyridine-2-carboxylate 1 g of ethyl 6-iodoimidazo[1,2-a]pyridine-2-carboxylate, 350 mg of tetrakis(triphenyl-phosphine)palladium(0) and 360 mg of lithium chloride are degassed under vacuum and then suspended, under argon, in 15 mL of degassed dioxane. After addition of 5 g of <strong>[145214-05-7]2-(tri-n-butylstannyl)oxazole</strong>, the reaction mixture is heated at 90 C. for 3.5 hours and then cooled, diluted and stirred with a mixture of 100 mL of aqueous 1M potassium fluoride solution and 200 mL of ethyl acetate. The aqueous phase is extracted with 200 mL of ethyl acetate and the combined organic phases are washed with brine and dried over sodium sulfate, filtered and concentrated to dryness under reduced pressure. The residue is chromatographed on silica, eluding with a gradient of ethyl acetate and hexane (from 80/20 to 100/0). The fractions containing the expected product are combined and concentrated to dryness under reduced pressure to give 530 mg of ethyl 6-(oxazol-2-yl)imidazo[1,2-a]pyridine-2-carboxylate in the form of a yellow powder. 1H NMR spectrum (DMSO-d6, δ in ppm): 9.30 (d, J=0.8, 1H), 8.68 (s, 1H), 8.30 (s, 1H), 7.85 (dd, J=1.7, 9.5, 1H), 7.79 (d, J=9.5, 1H), 7.44 (d, J=0.6, 1H), 4.33 (q, J=7.0, 2H), 1.33 (t, J=7.1, 3H). Mass spectrum (APCI): m/z=258 [M+H]+. |
|
With lithium chloride;tetrakis(triphenylphosphine) palladium(0); In 1,4-dioxane; at 90℃; for 3.5h;Inert atmosphere; |
1 g of ethyl 6-iodoimidazo[1,2-a]pyridine-2-carboxylate, 350 mg of tetrakis(triphenylphosphine)palladium(0) and 360 mg of lithium chloride are degassed under vacuum and then suspended, under argon, in 15 mL of degassed dioxane. After addition of 5 g of <strong>[145214-05-7]2-(tri-n-butylstannyl)oxazole</strong>, the reaction mixture is heated at 90 C. for 3.5 hours, then cooled, diluted and stirred with a mixture of 100 mL of aqueous 1M potassium fluoride solution and 200 mL of ethyl acetate. The aqueous phase is extracted with 200 mL of ethyl acetate and the combined organic phases are washed with brine and dried over sodium sulfate, filtered and concentrated to dryness under reduced pressure. The residue is chromatographed on silica, eluding with a gradient of ethyl acetate and hexane (from 80/20 to 100/0). The fractions containing the expected product are combined and concentrated to dryness under reduced pressure to give 530 mg of ethyl 6-(oxazol-2-yl)imidazo[1,2-a]pyridine-2-carboxylate in the form of a yellow powder. 1H NMR spectrum (DMSO-d6, δ in ppm): 9.30 (d, J=0.8, 1H), 8.68 (s, 1H), 8.30 (s, 1H), 7.85 (dd, J=1.7, 9.5, 1H), 7.79 (d, J=9.5, 1H), 7.44 (d, J=0.6, 1H), 4.33 (q, J=7.0, 2H), 1.33 (t, J=7.1, 3H). Mass spectrum (APCI): m/z=258 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping