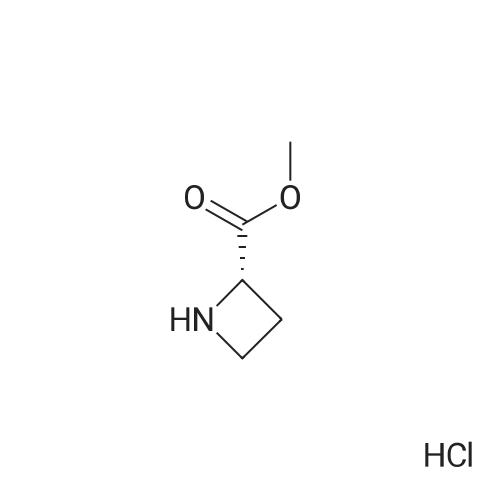
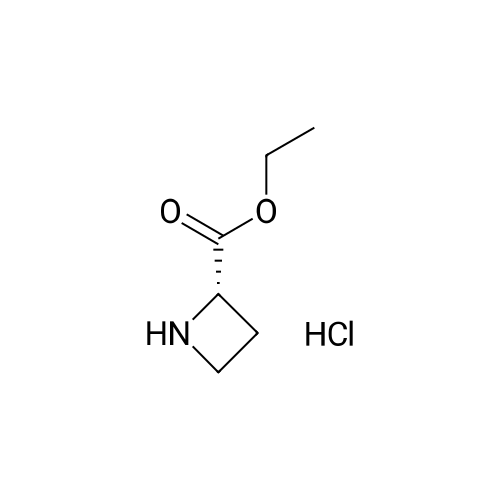
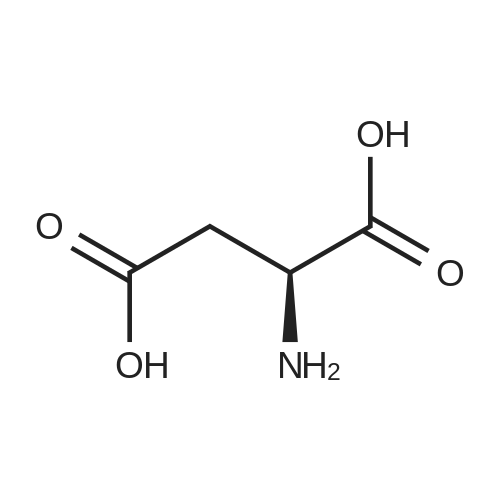
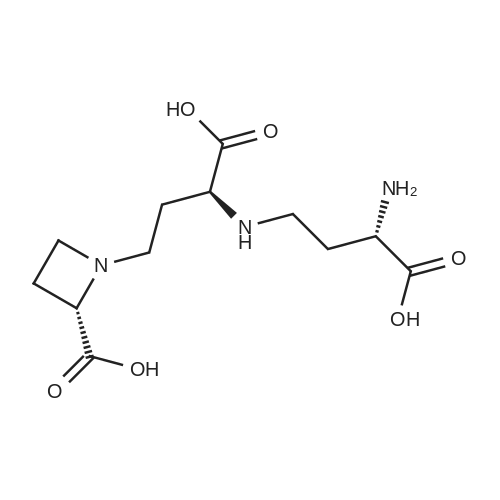
| 100% |
|
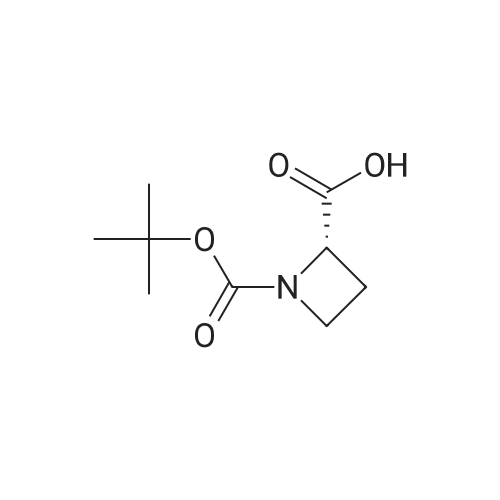
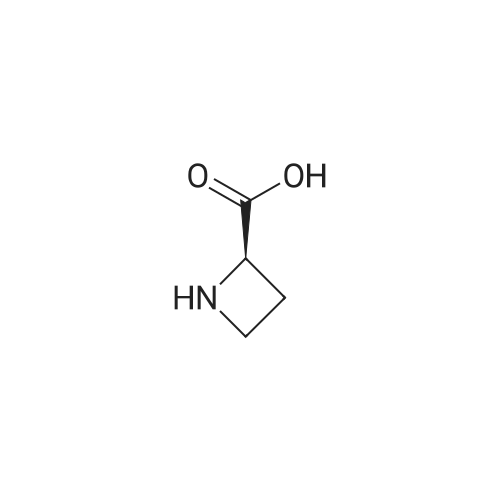
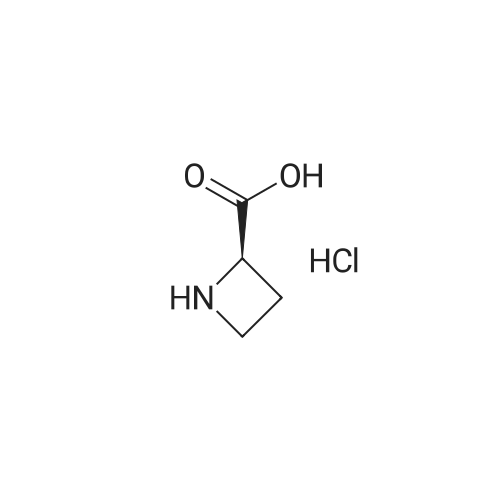
Example 15 (S)-1-(tert-Butoxycarbonyl)azetidine-2-carboxylic Acid (13). To a solution of (S)-2-azetidinecarboxylic acid 12 (1.0 g, 10.0 mmol) and di-tert-butyl dicarbonate (2.83 g, 12.5 mmol) in ethanol (20 mL) and water (10 mL) was added NaOH (420 mg, 10.5 mmol) at 0 C. The mixture was stirred overnight at ambient temperature. After evaporation of the ethanol, water (20 mL) was added, then acidified with diluted HCl to a pH of 3 and extracted with ethyl acetate (50 mL*3). The combined ethyl acetate was washed with water (30 mL) and saturated NaCl (30 mL), and dried over Na2 SO4. After evaporation of the ethyl acetate to afford 1.98 g (100%) of 13 as a white solid. 1H NMR (300 MHz, CDCl3) delta 4.79 (m, 1H), 3.93 (m, 2H), 2.46 (m, 2H), 1.48 (s, 9H). |
|
With tetramethyl ammoniumhydroxide; In acetonitrile; at 25℃; for 77h; |
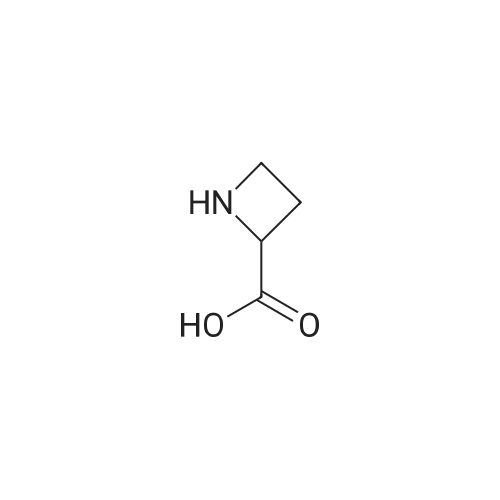
(a) (S)-1-(tert-Butyloxycarbonyl)azetidine-2-carboxylic acid.; A mixture of <strong>[2133-34-8]L-azetidine-2-carboxylic acid</strong> (1.0 g, 9.9 mmol, Toronto Research), tetramethylammonium hydroxide pentahydrate (2.0 g, 11 mmol, Aldrich) and di-tert-butyl dicarbonate (3.2 g, 15 mmol, Aldrich) in acetonitrile (50 mL) was stirred for 3 d at 25 C. Additional amount of di-tert-butyl dicarbonate (1.0 g, 4.6 mmol) was added and the mixture was stirred for 5 h at 25 C. The reaction mixture was evaporated under reduced pressure and the residue was partitioned between aqueous solution of K2CO3 and 1:1 mixture of EtOAc/hexane. The aqueous phase was separated, acidified with aqueous solution of citric acid, and extracted with EtOAc (3×). The combined extracts were washed with brine, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was dried under vacuo to afford the title compound as a white solid. MS (ESI, neg. ion.) m/z: 200 (M-1). |
|
With 4-methyl-morpholine; sodium hydrogencarbonate; In 1,4-dioxane; water; |
7a. 1-t-butyloxycarbonyl-2-(S)-azetidinecarboxylic acid To an ice cooled solution of 2-(S)-azetidinecarboxylic acid (10.15 g, 100.39 mmol) in 1,4 dioxane:water (300 mL, 1:1) was added di-tert-butyl dicarbonate (28.48 g, 130.51 mmol), followed by 4-methylmorpholine (11.68 g, 115.45 mmol). The reaction mixture continued to stir 18 hours, gradually warming to room temperature. The reaction mixture was then poured into a ice cooled saturated solution of sodium bicarbonate (250 mL) and washed with ethyl acetate (3*250 ml). The aqueous was then acidified with potassium hydrogen sulfate (pH=1) and the product extracted with ethyl acetate (3*300 ml). These extracts were then dried (Na2 SO4), filtered and concentrated in vacuo. The resulting semisolid was carried forward without further purification. MS (DCI/NH3) m/e 202 (M+H)+, 219 (M+NH4)+. 1 H NMR (CDCl3, 300 MHz) delta: 10.0 (br s, 1H), 4.81-4.76 (t, J=15 Hz, 1H), 3.99-3.83 (m, 2H), 2.62-2.38 (m, 2H), 1.48 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping