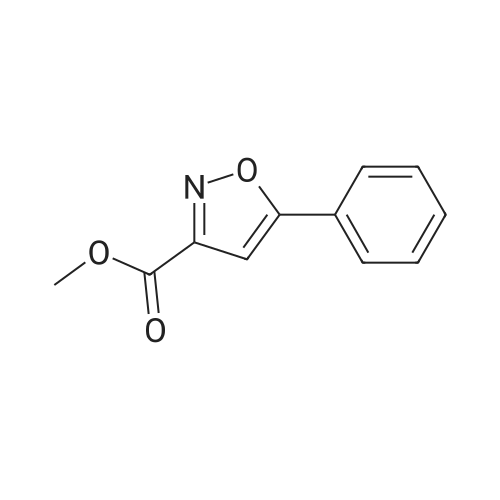
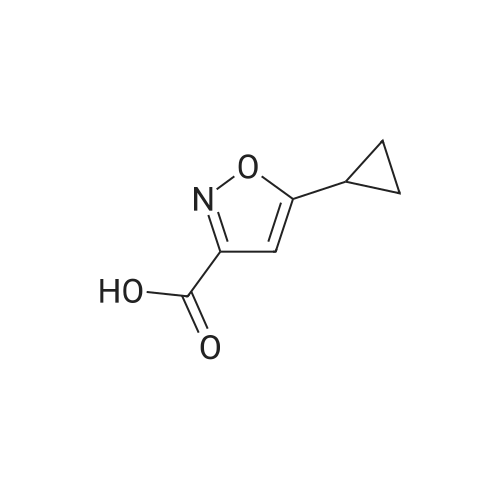
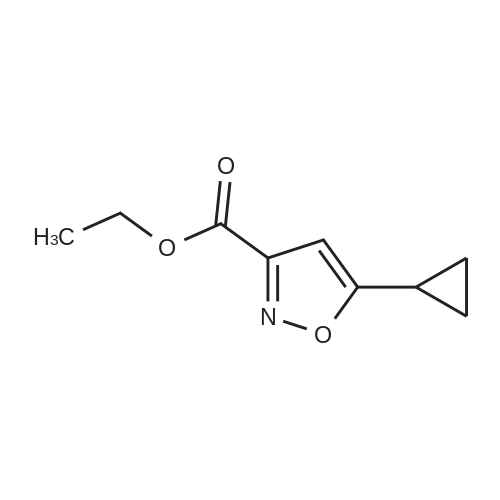
| 84% |
With hydroxylamine hydrochloride; In ethanol; for 1h;Heating / reflux; |
Sodium ethoxide (10 g, 147 mmol) is combined with absolute EtOH (60 mL) in an oven-dried flask, under nitrogen and heated to 70° C. to aid dissolution. The mixture is cooled to 0° C., treated drop-wise with a mixture of cyclopropyl methyl ketone (14.56 mL, 147 mmol) and diethyl oxylate (19.96 mL, 147 mmol) and warmed to RT. Stirring was difficult, so additional EtOH (60 mL) is added and the mixture is stirred for 1 h, then heated to 80° C. for 45 min. The mixture is cooled to RT and concentrated to dryness. The resulting solid is triturated with EtOAc, filtered, and rinsed with EtOAc and Et2O to remove the reddish color. The solid is dissolved in water (300 mL), acidified to pH 2 with dilute H2SO4, extracted with Et2O (400 mL total), dried (Na2SO4) and concentrated to afford 18.0 g (66percent yield) of <strong>[21080-80-8]ethyl 4-cyclopropyl-2,4-dioxobutanoate</strong> as an amber oil. HRMS (ESI) calcd for C9H12O4 +H: 185.0814, found 185.0821 (M+H)+. Ethyl 4-cyclopropyl-2,4-dioxobutanoate (12.92 g, 70.1 mmol) is combined with hydroxylamine hydrochloride (14.62 g, 210.4 mmol) in EtOH (250 mL), heated to reflux for 1 h, cooled, and concentrated to dryness. The residue is partitioned between H2O (250 mL) and EtOAc (2.x.250 mL) and the combined organics are dried (MgSO4) and concentrated to an amber oil (13.89 g). The crude material is chromatographed over 500 g silica gel, eluting with 25percent EtOAc/hexane. The appropriate fractions are combined and concentrated to afford 10.71 g (84percent yield) of ethyl 5-cyclopropylisoxazole-3-carboxylate as a yellow oil. MS (CI) m/z: 182 (M+H)+. Sodium hydroxide (1.76 g, 44.0 mmol) in H2O (5 mL) is added to a solution of ethyl 5-cyclopropylisoxazole-3-carboxylate (1.97 g, 10.9 mmol) in MeOH (10 mL). The mixture is stirred at RT for 3 h, concentrated to remove the MeOH, and acidified to pH 2 with 5percent HCl. The acid is extracted with CH2Cl2 (6.x.20 mL), dried (MgSO4) and concentrated to afford 1.56 g (93percent yield) of 5-cyclopropylisoxazole-3-carboxylic acid as a white solid. MS (Cl) m/z: 154 (M+H)+. 5-Cyclopropylisoxazole-3-carboxylic acid (1.53 g, 10 mmol) is dissolved in benzene (30 mL), treated with oxalyl chloride (3.46 mL, 40 mmol) and heated to reflux for 2 h. The mixture is cooled, concentrated to dryness and the residual benzene is azeotroped off with CH2Cl2. The resulting acid chloride is dissolved in Me2CO (15 mL) and treated with a solution of NaN3 (1.95 g, 30 mmol) in H2O (7 mL). The mixture is vigorously stirred for 1 h, concentrated to remove the Me2CO, triturated with H2O, filtered, rinsed with water and dried under vacuum to afford 1.76 g (99percent yield) of 5-cyclopropylisoxazole-3-carbonyl azide as an off-white solid. 1H NMR (CDCl3, 400 MHz): delta 1.02, 1.14, 2.10, 6.35 ppm. 5-Cyclopropylisoxazole-3-carbonyl azide (447 mg, 2.5 mmol) is combined with 5-chloro-2,4-dimethoxyaniline (471 mg, 2.5 mmol) in anhydrous MeCN (30 mL) and heated to reflux for 18 h. The mixture is cooled and the resulting solid is filtered, rinsed with Et2O and dried in a vacuum oven to afford 619 mg (73percent yield) of Example 621 as a very light purple solid. HRMS (ESI) calcd for C15H16N3O4Cl +H: 338.0907, found 338.0896 (M+H)+. |
| 66.3% |
With hydroxylamine hydrochloride; In ethanol; at 20 - 80℃; for 2h; |
Into a 10 L round-bottom flask, was placed a solution of ethyl 4-cyclopropyl-2,4- dioxobutanoate (177 g) in ethanol (1.1 L) and NH2OH-HCl (200 g). The resulting solution was stirred for 1 h at 20-30°C. The resulting solution was allowed to react, with stirring, for an additional 1 h at 80°C. The resulting mixture was concentrated under vacuum. The residue was purified on a silica gel column with ethyl acetate/petroleum ether (1/10). This resulted in 143 g (the two step yield was 66.3percent) of ethyl 5- cyclopropylisoxazole-3-carboxylate as a yellow oil. TLC (ethyl acetate/petroleum ether = l/5): Rf = 0.2. |
| 143 mg |
With hydroxylamine hydrochloride; In ethanol; water; at 20 - 80℃; for 2h; |
Into a 10 L round-bottom flask, was placed a solution of ethyl 4-cyclopropyl-2,4- dioxobutanoate (177 g) in ethanol (1.1 L) and NH2OH-HCl (200 g). The resulting solution was stirred for 1 h at 20-30°C. The resulting solution was allowed to react, with stirring, for an additional 1 h at 80°C. The resulting mixture was concentrated under vacuum. The residue was purified on a silica gel column with ethyl acetate/petroleum ether (1/10). This resulted in 143 g (the two step yield was 66.3percent) of ethyl 5- cyclopropylisoxazole-3-carboxylate as a yellow oil. TLC (ethyl acetate/petroleum ether =1/5): Rf = 0.2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping