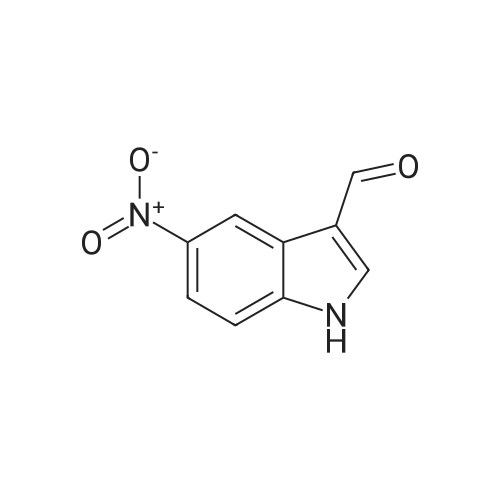
| 84% |
With palladium 10% on activated carbon; hydrogen; In ethanol; for 4h; |
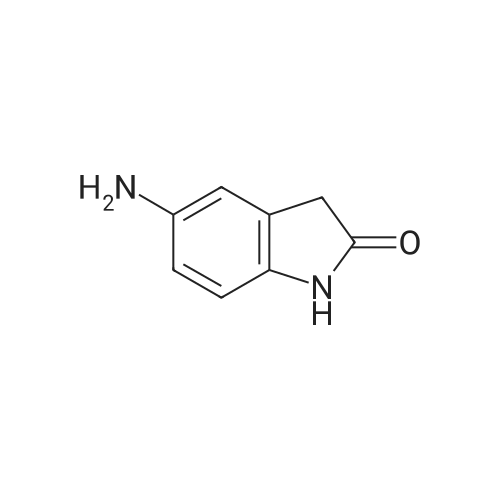
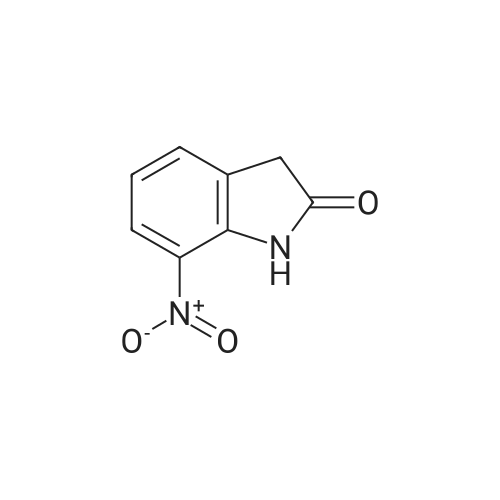
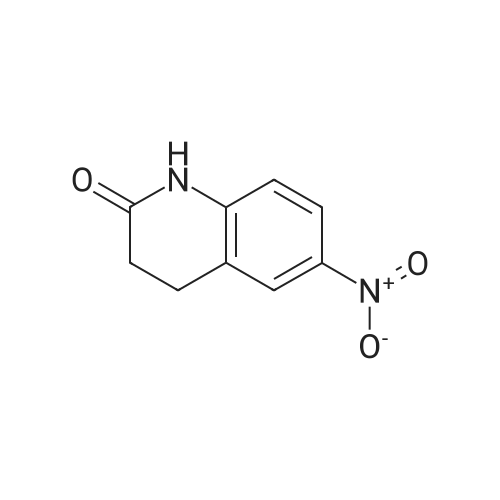
The commercial 5-nitroindolin-2-one (1.00 g, 5.61 mmol) was hydrogenated in EtOH (70 mL) in the presence of 10% Pd-C (315 mg, 2.97 mmol) for 4 h. Then the catalyst was filtered off through Celite, the Celite rinsed with additional EtOH and the solution was evaporated, to give 17 as a brown solid (698 mg, 4.71 mmol, 84% yield). 1H NMR (CD3OD): delta 3.43 (s, 2H, CH2); 6.63-6.65 (m, 2H, Ar); 6.73-6.74 (m, 1H, Ar) ppm. Anal. (C8H8N2O) Calc%: C 64.85, H 5.44, N 18.91; Found%: C 65.03, H 5.49, N 18.83. |
| 84% |
With palladium 10% on activated carbon; hydrogen; In ethanol; at 20℃; for 4h; |
The 5-nitro-2-oxindole 1 (1 .00 g, 5.61 mmol) was hydrogenated in EtOH (70 mL)in the presence of 10% Pd-C (315 mg, 2.97 mmol) for 4h at room temperature.Then the catalyst was filtered off through Celite, the Celite rinsed with additional EtOH and the solution was evaporated, to give 2 as a brown solid (698 mg, 4.71 mmol, 84% yield).1HNMR (400 MHz, DMSO-d6): 6 3.30 (5, 2H, CH2); 4.62 (br s, 2H, NH2); 6.37(dd, 1H, J= 2.2, 8.2 Hz, Ar); 6.48-6.50 (m, 2H, Ar); 9.90 (br s, 1H, NH) ppm.Anal. Calcd for C8H8N20: C, 64.85%; H, 5.44%; N 18.91%; Found: C, 65.03%;H, 5.49%; N, 18.83% |
| 79% |
With hydrogenchloride; zinc; In ethanol; water; for 0.5h; |
To the mixture of 5-nitro-2-oxindole 2c (0.5g, 0.003mol) and zinc dust (0.91g, 0.014mol) in ethanol (5mL) 2mL of concentrated hydrochloric acid were rapidly added with intensive stirring. After 30min the reaction was terminated by addition of cold water (3mL). Then solid sodium bicarbonate was carefully added to the reaction mixture to adjust pH 8. Solvent was removed in vacuum and crude product was purified by filtering through silica gel pad (eluent MeOH:EtOAc 1:1). After eluent evaporation in vacuum the 5-amino-2-oxindole 2d was obtained as gray powder with yield 79% (0.35g) and used without additional purification. Mp (exp)=190-195C, mp lit34=212-214C). dH (400MHz, DMSO-d6): 3.31 (2H, s, CH2), 4.64, (1H, br s, NH2) 6.39 (1H, dd, J 8.1, 1.3, CHAr), 6.51 (1H, s, CHAr), 6.53 (1H, d, J 7.6, CHAr), 9.99 (1H, s, NH). |
| 69% |
With hydrogen;palladium on activated charcoal; In acetic acid; under 2068.65 Torr; for 1.5h; |
To 250 mL acetic acid was added 7.00 g (39.3 MMOL) 5-nitro-1, 3-dihydro-indol-2-one and 418 mg (0.393 MMOL) palladium on carbon. Exposed the reaction mixture to 40 psi H2 on parr shaker for 1.5 hours. The reaction was filtered through diatameceous earth, and the acetic acid was removed under reduced pressure. Cooled the reaction mixture to 0 C and added 10.0 mL of a 94.5 : 5: 0.5 CHC13 : CH30H: NH40H solution. The solution was loaded onto a silica gel column and purified via chromatography (97.8 : 2.0 : 0.2 CHCI3 : CH30H: NH40H) to give a white solid which was further crystallized using the eluent as the solvent to give 4.06 G (27.2 mmol, 69%) of the title compound as crystalline white needles. C8H9N20 : |
| 68.9% |
With hydrogen;5% Pd(II)/C(eggshell); In acetic acid; at 20℃; |
Step 2 5-Amino-1,3-dihydro-indol-2-one 5-Nitro-1,3-dihydro-indol-2-one 30b (3.56 g, 20 mmol) was dissolved in 200 ml of acetic acid under stirring, and added with palladium on activated carbon (1.0 g, 5%) to the solution at room temperature. The reaction mixture was stirred under a hydrogen atmosphere. After thin lay chromatography showed the disappearance of starting materials, the reaction mixture was filtered, and concentrated under reduced pressure to obtain the title compound 5-amino-1,3-dihydro-indol-2-one 30c (2.04 g, yield 68.9%) as a white solid. |
| 68.9% |
With hydrogen;5% Pd(II)/C(eggshell); In acetic acid; at 20℃; |
5-Nitro-1,3-dihydro-indol-2-one 30b (3.56 g, 20 mmol) was dissolved in 200 ml of acetic acid under stirring, and added with palladium on activated carbon (1.0 g, 5%) to the solution at room temperature. The reaction mixture was stirred under a hydrogen atmosphere. After thin lay chromatography showed the disappearance of starting materials, the reaction mixture was filtered, and concentrated under reduced pressure to obtain the title compound 5-amino-1,3-dihydro-indol-2-one 30c (2.04 g, yield 68.9%) as a white solid. MS m/z (ESI): 149.4[M+1] |
| 60% |
palladium; In methanol; |
5-Amino-2-Oxindole 5-Nitro-2-oxindole (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of the title compound as a white solid. |
| 60% |
palladium; In methanol; |
5-Nitro-2-oxindole (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of 5-amino-2-oxindole as a white solid. |
| 60% |
palladium; In methanol; |
5-Amino-2-oxindole 5-Nitro-2-oxindole (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of the title compound as a white solid. |
| 60% |
palladium; In methanol; |
5-Amino-2-oxindole 5-Nitro-2-oxindole (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of the title compound as a white solid. |
| 60% |
palladium; In methanol; |
(c) Preparation of 5-Amino-2-oxindole. The 5-nitro-2-oxindole (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of the title compound as a white solid. |
| 60% |
palladium; In methanol; |
5-Nitro-1,3-dihydro-indol-2-one (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of 5-amino-1,3-dihydro-indol-2-one as a white solid. |
| 60% |
With hydrogen;palladium 10% on activated carbon; In methanol; |
5-Amino-2-oxindole 5-Nitro-2-oxindole (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of the title compound as a white solid, |
| 60% |
With hydrogen;palladium 10% on activated carbon; In methanol; |
5-Amino-2-oxindole 5-Nitro-2-oxindole (6.3 g) was hydrogenated in methanol over 10% palladium on carbon to give 3.0 g (60% yield) of the title compound as a white solid. |
| 50% |
With hydrogen;palladium 10% on activated carbon; In ISOPROPYLAMIDE; under 2068.65 Torr; for 16h;Parr bottle; |
Preparation of 5-amino-oxindole (2) To a solution of 5-nitro-oxindole (25 g) in 120 mL of dimethylacetamide in a Parr bottle was added 10% Pd/C (0.5 g). The mixture was hydrogenated (40 psi H2) for 16 hours. The catalyst was removed by filtration and the filtrate was diluted with ether (2 L) to provide 5-amino-oxindole (10.5 g; 50%). |
| 50% |
With hydrogen;palladium 10% on activated carbon; In ISOPROPYLAMIDE; under 2068.65 Torr; for 16h; |
To a solution of 5-nitro-oxindole (25 g) in 120 mL of dimethylacetamide in a Parr bottle was added 10% Pd/C (0.5 g). The mixture was hydrogenated (40 psi H2) for 16 hours. The catalyst was removed by filtration and the filtrate was diluted with ether (2 L) to provide 5-amino-oxindole (10.5 g; 50%). |
|
With hydrogen;palladium 10% on activated carbon; In methanol; under 2327.23 Torr; for 2h; |
A. A suspension of 5-nitroindolin-2-one (2.0 g, 11.13 mmol), and 10% palladium on carbon (500 mg) in methanol (100 mL) was hydrogenated for 2 hours under 45 psi. The reaction mixture was filtered through celite and the resulting cake was washed with methanol. The filtrate was concentrated to afford 5-aminoindolin-2-one as a tan-colored solid (1.6 g). |
|
With palladium on activated charcoal; hydrogen; In ethanol; at 20℃; |
The derivatives 1 -5 were synthesized following the synthetic procedure reported in scheme 1 of Figure 1 . The 5-nitro-1 ,3-dihydro-2/-/-indol-2-one commercial compound was reduced to the corresponding 5-amino-1 ,3-dihydro-2H-indol-2-one by means of catalytic hydrogenation using Pd/C as a catalyst. The subsequent reaction with the chloro acetyl chloride provided 2-chloro-/V-(2-oxo-2,3-dihydro-1 /-/-indol-5-yl)acetamide which was condensed with the appropriate amine derivative to provide, respectively, 2-[(6,7-dimethoxy-3,4-dihydroisoquinol-2-(1 H)-yl)]-N-(2-oxo-2,3- dihydro-1 H-indol-5-yl)acetamide and 2-[(3,4-dimethoxybenzyl)amino]-/V-(2-oxo- 2,3-dihydro-1 /-/-indol-5-yl)acetamide. Lastly, condensation of the appropriate aromatic carbaldehyde in the presence of pyrrolidine provided the desired products 1 -5. |
|
With iron; ammonium chloride; In ethanol; water; for 2h;Reflux; |
General procedure: To a suspension of 5-nitro-3-substituted-2-oxindole derivatives 8-12 or commercially available 5-nitroindolin-2-one (1 mmol) in amixture of ethanol and water (10 mL, 4:1 v/v) was added iron powder (560 mg.10 mmol,10 equiv.) and NH4Cl (134 mg, 2.5 mmol,2.5 equiv.). The mixture was refluxed for 2 h, cooled and filteredthrough Celite. The filtrate was diluted with water (5 mL) andextracted with dichloromethane (3 x 10 mL). The combinedorganic layers were washed with water (10 mL) and brine (10 mL),dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude residue was purified by crystallization withethyl ether. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping