Synthesis and biological evaluation of structurally diverse 6-aryl-3-aroyl-indole analogues as inhibitors of tubulin polymerization
Wen Ren
;
Yuling Deng
;
Jacob D. Ward
, et al.
Eur. J. Med. Chem.,2024,263,115794.
DOI:
10.1016/j.ejmech.2023.115794
More
Abstract: The synthesis and evaluation of small-molecule inhibitors of tubulin polymerization remains a promising approach for the development of new therapeutic agents for cancer treatment. The natural products colchicine and combretastatin A-4 (CA4) inspired significant drug discovery campaigns targeting the colchicine site located on the beta-subunit of the tubulin heterodimer, but so far these efforts have not yielded an approved drug for cancer treatment in human patients. Interest in the colchicine site was enhanced by the discovery that a subset of colchicine site agents demonstrated dual functionality as both potent antiproliferative agents and effective vascular disrupting agents (VDAs). Our previous studies led to the discovery and development of a 2-aryl-3-aroyl-indole analogue (OXi8006) that inhibited tubulin polymerization and demonstrated low nM IC50 values against a variety of human cancer cell lines. A water-soluble phosphate prodrug salt (OXi8007), synthesized from OXi8006, displayed promising vascular disrupting activity in mouse models of cancer. To further extend structure-activity relationship correlations, a series of 6-aryl-3-aroyl-indole analogues was synthesized and evaluated for their inhibition of tubulin polymerization and cytotoxicity against human cancer cell lines. Several structurally diverse molecules in this small library were strong inhibitors of tubulin polymerization and of MCF-7 and MDA-MB-231 human breast cancer cells. One of the most promising analogues (KGP591) caused significant G2/M arrest of MDA-MB-231 cells, disrupted microtubule structure and cell morphology in MDA-MB-231 cells, and demonstrated significant inhibition of MDA-MB-231 cell migration in a wound healing (scratch) assay. A phosphate prodrug salt, KGP618, synthesized from its parent phenolic precursor, KGP591, demonstrated significant reduction in bioluminescence signal when evaluated in vivo against an orthotopic model of kidney cancer (RENCA-luc) in BALB/c mice, indicative of VDA efficacy. The most active compounds from this series offer promise as anticancer therapeutic agents.
Keywords:
Inhibitors of tubulin polymerization ;
Vascular disrupting agents ;
Indole synthesis ;
Molecular docking ;
Antiproliferative agents ;
Inhibitors of cell migration
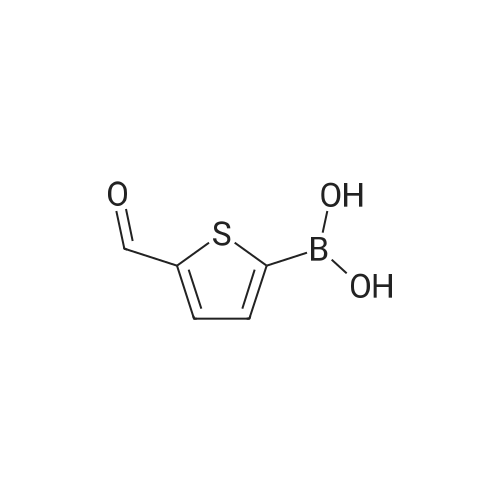
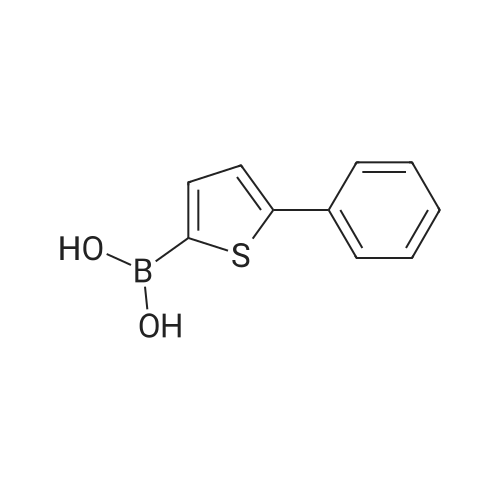
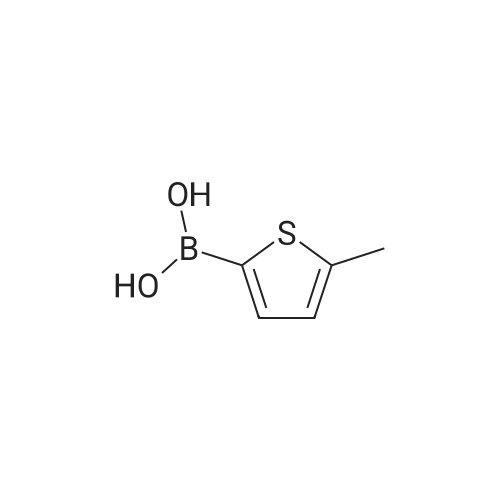
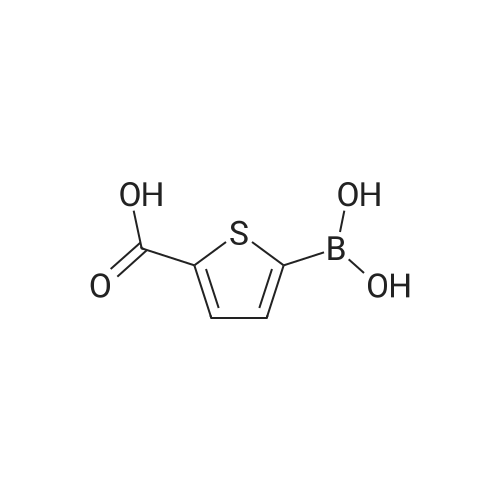
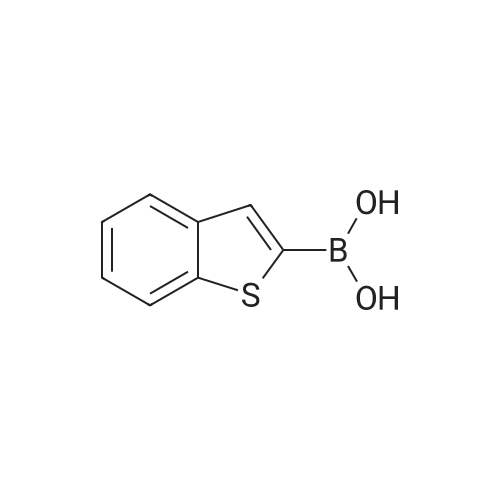
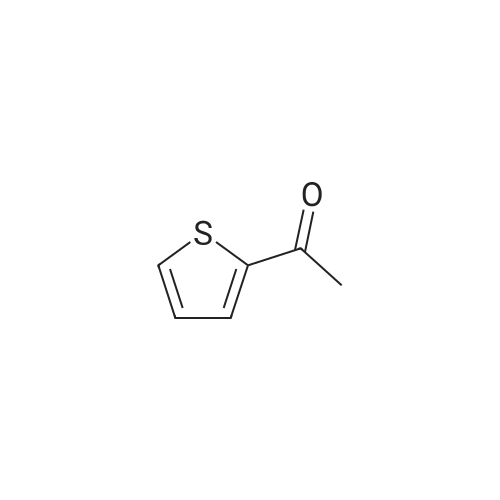
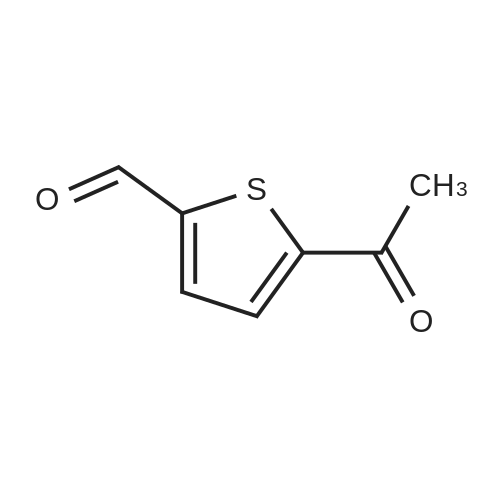
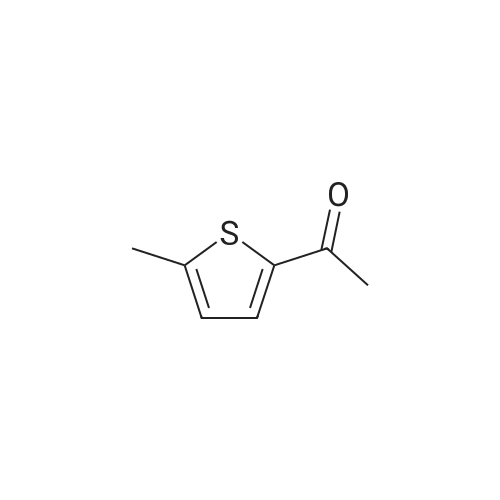
Purchased from AmBeed:
128796-39-4 ;
10365-98-7 ;
98-80-6 ;
98437-24-2 ;
5720-05-8 ;
64-86-8 ;
13331-27-6 ;
206551-43-1 ;
63139-21-9 ;
622864-48-6 ;
5720-07-0 ;
87199-18-6 ;
30418-59-8 ;
4521-61-3 ;
4521-61-3 ;
87199-18-6 ;
64-86-8 ;
64-86-8 ;
128796-39-4 ;
5720-05-8 ;
64-86-8
...More

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping