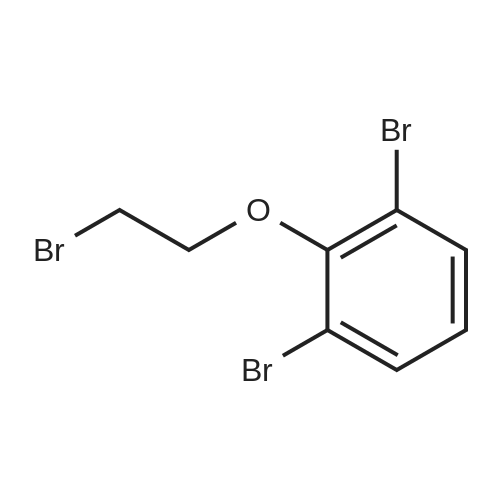
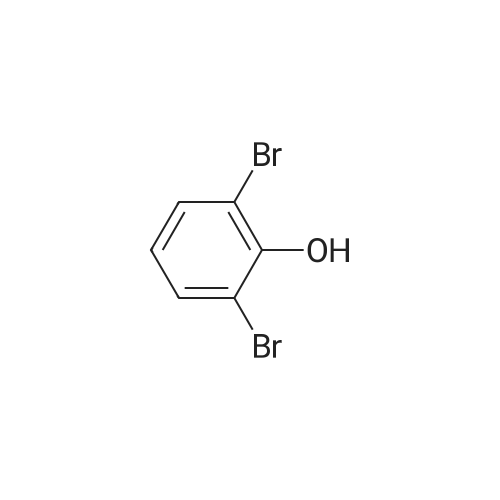
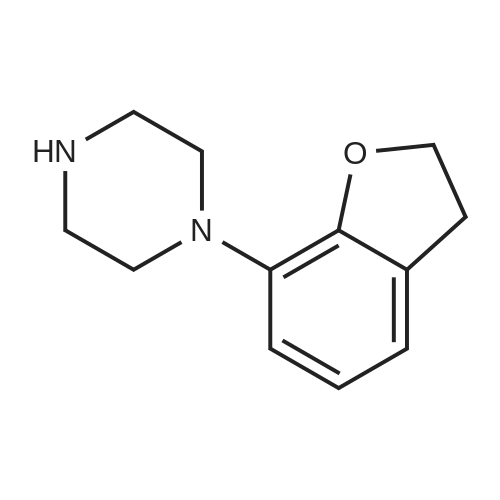
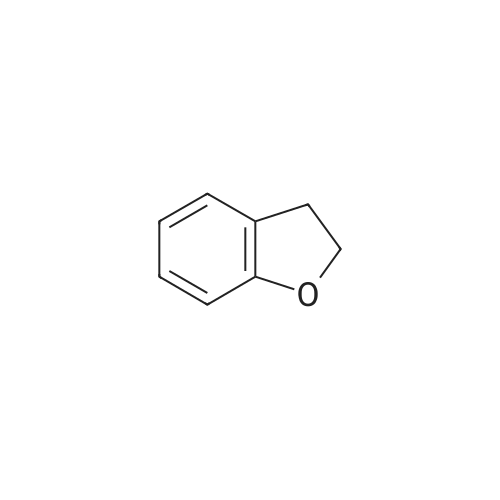
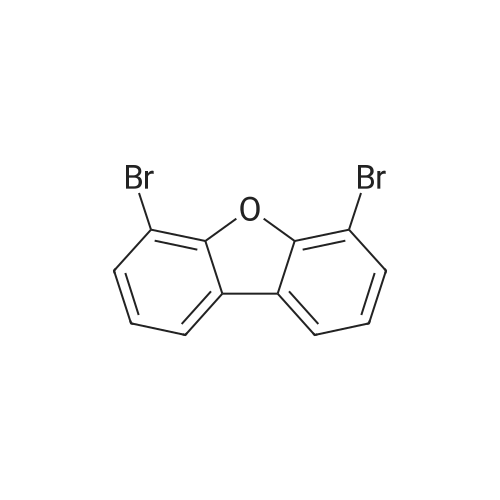
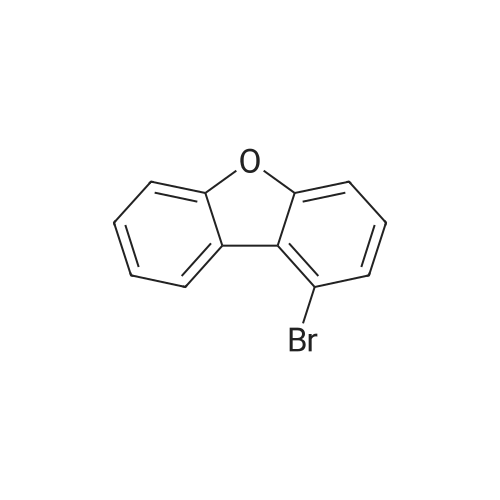
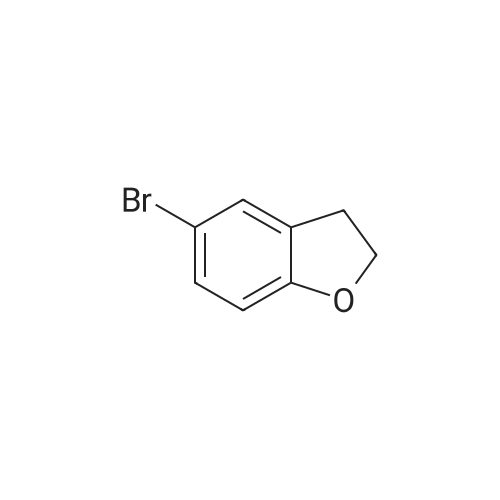
| 78% |
With n-butyllithium; In tetrahydrofuran; hexane; at -78 - 0℃; for 1h; |
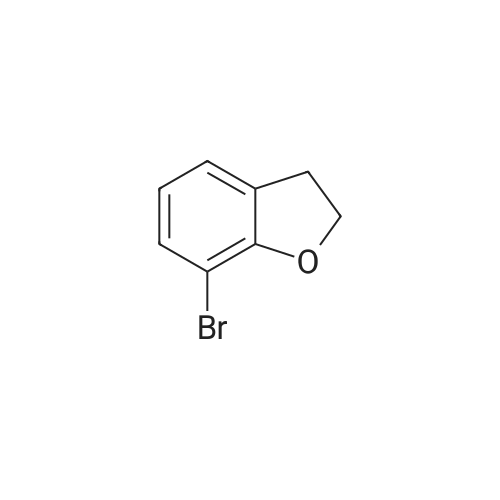
Synthesis of 7-Bromo-2,3-dihydro-benzofuran A solution of 2.5M n-BuLi (13.0 ml, 32.5 mmol) was added to a solution of 1,3-dibromo-2-(2-bromo-ethoxy)-benzene (11.5, 32.0 mmol) in 115 ml of THF and 28 ml of hexane at -78 C. over 30 mins. The reaction was continued at -78 C. for 30 minutes, then warmed to 0 C. The mixture was poured into water (100 ml) and the aqueous phase was extracted with ether. The combined organic layers were dried over Na2SO4, filtered, and concentrated to give a pale yellow oil. Silica gel chromatography using a gradient of ethyl acetate in hexanes to give 7-bromo-2,3-dihydro-benzofuran as colorless needles (5.00 g, 78%). 1H NMR (500 MHz, DMSO-d6) delta 7.27(dd, 1 Hz, 8 Hz, 1H), 7.20 (dd, 1 Hz, 7.5 Hz, 1H), 6.75 (t, 7.8 Hz, 1H), 4.59 (t, 9 Hz, 2H), 3.28 (t, 8.8 Hz, 2H). |
|
With n-butyllithium; In tetrahydrofuran; hexane; at -80℃; |
1-(2,3-Dihydrobenzofuran-7-yl)piperazine (C7i); In detail, for the synthesis of compound C7i 2,6-dibromophenole (29 mmol) (C8a; R4-R6 = H) is mixed with 1,2-dibromoethane (29 mmol) (r = 1) unter basic aqueous conditions (NaOH) and heated at reflux for 18 hrs. The resulting monoalkylation product can be solved in THF/hexane (4/1), cooled to -80 C and then, a solution of 2,5 M butyllithium (18mmol) in hexane is added dropwise (17.1 mmol). The cyclisation product 7-bromo-2,3-dihydrobenzofurane (4 mmol) (C8b; r = 1) can be suspended together with NaOtBu (20 mmol), Pd2(dba)3 (2 mol%), BINAP (2 mol%) and piperazine (8 mmol) in 5 ml dry toluene and heated at 117C for 6 hrs to get the product. |
|
With n-butyllithium; In tetrahydrofuran; at -100℃; |
2. Synthesis of 2,3-dihydrobenzofuran-7-sulfonyl chloride; n-Butyllithium (23 mmol) was added dropwise to a solution of 1,3-dibromo-2-(2-bromoethoxy)benzene (21.8 mmol) in tetrahydrofuran (100 mL) at -100 C. and the reaction mixture was maintained for 30 min. n-Butyllithium (23 mmol) was added dropwise and the reaction mixture was maintained at -100 C. for an additional 60 min. Sulfur dioxide (43.8 mmol) was added and the reaction mixture was maintained for 2 h between -100 and -85 C. The reaction mixture was diluted with hexane (100 mL) and the precipitated solids were collected by filtration. The solid was suspended in dichloromethane (100 mL) at 0 C. and N-chlorosuccinamide (24.6 mmol) was added in several batches. The reaction mixture was maintained for 60 min at 0 C. and was diluted with dichloromethane (100 mL). The reaction mixture was washed with (2 M) sodium hydrogen sulfate (2×150 mL) and brine (3×100 mL), was dried (sodium sulfate), and was concentrated. The residue was purified by Flash chromatography (1/50 ethyl acetate/petroleum ether) to provide 2,3-dihydrobenzofuran-7-sulfonyl chloride in 51% yield as a light yellow solid. Data: 1H NMR: (CDCl3) delta 3.35 (t, 2H), 4.92 (t, 2H), 6.96 (t, 1H), 7.54 (s, 1H), 7.64 (d, 1H). LC/MS (ES) m/z 283 [C13H18N2O3S+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping