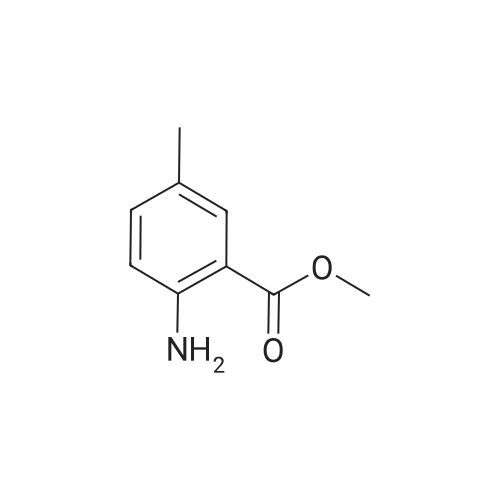
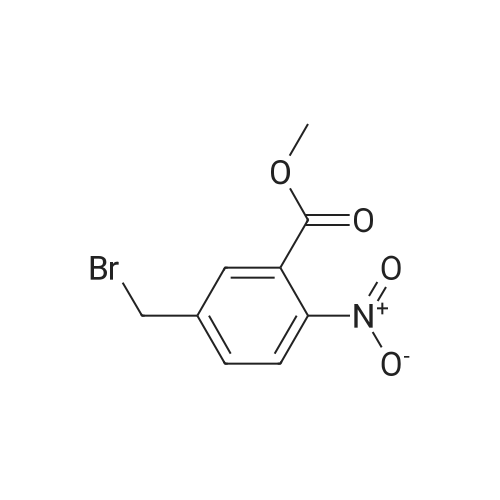
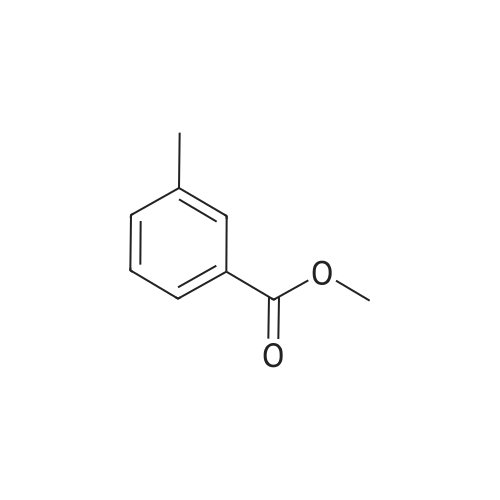
| 44.0% |
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; 2,2'-azobis(isobutyronitrile); In acetic acid methyl ester; at 75 - 78℃; for 13.6667h; |
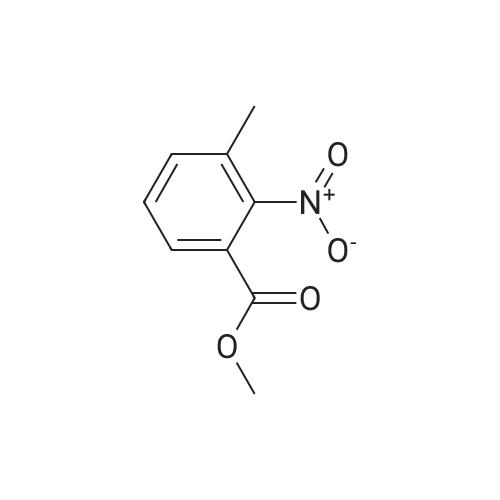
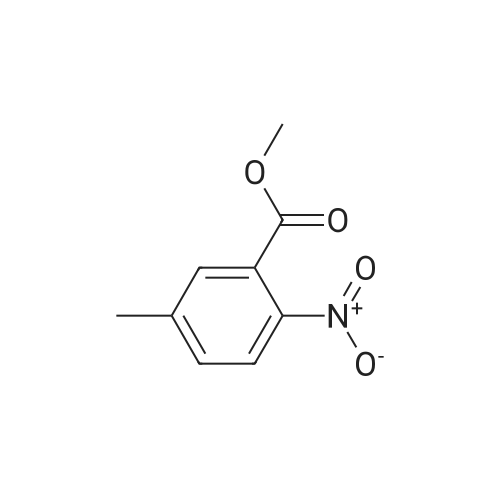
Step 1: A mixture of <strong>[20587-30-8]5-methyl-2-nitro-benzoic acid methyl ester</strong> (93.95 g, 481.35 mmol), 1,3-dibromo-5,5-dimethylhydantoin (75.70 g, 264.74 mmol), in methyl acetate (550 mL) was heated at 78 C. for 40 minutes while stirred with a mechanical stirrer. Then a solution of 2,2'-azobisisobutyro-nitrile (3.95 g, 24.07 mmol) in methyl acetate (80 mL) was added, and the mixture was heated at about 75 C. for 13 hours. The mixture was allowed to cooled to 15 C. and stirred for 2 hours to age the precipitate. The suspension was filtered, washed with 10 C. methyl acetate (2*50 mL) to give a brown filtrate. To the filtrate, was added heptane (500 mL). The organic layer was washed with 2% brine (2*500 mL) and water (2*500 mL), and concentrated to about 2 volumes. To the mixture, was added t-butyl methyl ether (or MTBE, 300 mL). The mixture was heated at about 70 C. for 15 minutes, cooled to about 53 C. over one hour, seeded with the product (about 250 mg, or simply re-crystallized) at 45 C., then at 20-25 C., while blowing nitrogen with a glass pipette overnight. The resulting solid was filtered via a medium pore-sized funnel, washed with a pre-cooled 10 C. mixed solvent of heptane/MTBE (1/2 vol/vol) and suction dried in hood overnight to give 5-bromomethyl-2-nitro-benzoic acid methyl ester as an off-white solid (58.3 g, 44.0% yield). The solid was used in the next step without further purification. |
| 40% |
With N-Bromosuccinimide; dibenzoyl peroxide; In chloroform;Reflux; |
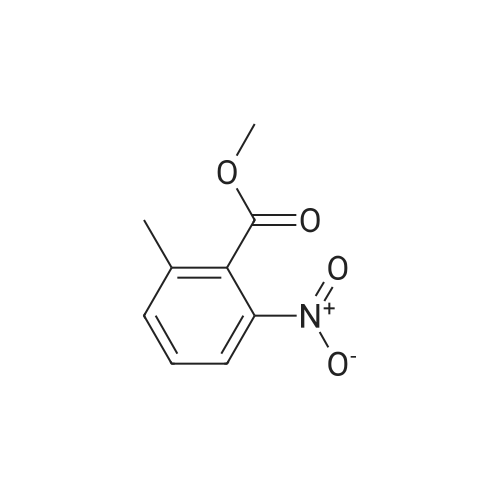
Example 11. Preparation of N-((2-(4-hydroxy-3,5-dimethylphenyl)-4-oxo-3,4-dihydroquinazolin-6-yl)methyl)methanesulfonamide [0184] To a solution of <strong>[20587-30-8]methyl-5-methyl-2-nitrobenzoate</strong> (2.3 g, 11.8 mmol) in CHCI3 (150 mL) was added NBS (5.3 g, 30.0 mmol) and benzoyl peroxide (0.285 g, 1.2 mmol). The reaction was heated at reflux temperature overnight. Then, the resulting mixture was washed sequentially with H2O, Na2CO3, and brine. The organic layer was then dried (Na2SO4), filtered, and concentrated in vacuo. Purification by flash chromatography on silica gel, eluting with 5% to 20% ethyl acetate/heptane, afforded methyl 5-(bromomethyl)-2-nitrobenzoate (1.3 g, 40%).[0185] To a solution of methyl 5-(bromomethyl)-2-nitrobenzoate (1.3 g, 4.7 mmol) in DMF (15 mL) was added potassium phthalimide (1.0 g, 5.2 mmol) and the reaction was stirred at room temperature for 1 hour and concentrated in vacuo. Purification by flash chromatography, eluting with 15% to 70% ethyl acetate/heptane, afforded methyl 5-((1,3-dioxoisoindolin-2-yl)methyl)-2-nitrobenzoate (1.4 g, 88%).[0186] A solution of methyl 5-((1 ,3-dioxoisoindolin-2-yl)methyl)-2- nitrobenzoate (0.50 g, 1.4 mmol) in EtOH (10 mL) was treated with hydrazine (0.14 mL, 4.4 mol) and the reaction was stirred at room temperature overnight. After this time, the mixture was concentrated in vacuo and purified by flash chromatography on silica gel, eluting with 30% to 100% of 92:7:1 CHCb/MeOH/concentrate NH4OH in CH2CI2, to afford methyl 5-(aminomethyl)-2-nitrobenzoate (0.23 g, 78%).[0187] To a solution of methyl 5-(aminomethyl)-2-nitrobenzoate (0.23 g, 1.1 mmol) in CH2CI2 (5 ml_) was added Et3N (0.31 ml_, 2.2 mmol) and methanesulfonyl chloride (0.08 ml_, 1.1 mmol). The reaction was stirred for 15 minutes at room temperature, concentrated in vacuo, and purified by flash chromatography on silica gel, eluting with 2% to 20% MeOH/CH2CI2, to afford methyl 5-(methylsulfonamidomethyl)-2-nitrobenzoate (0.18 g, 57%).[0188] A mixture of methyl 5-(methylsulfonamidomethyl)-2-nitrobenzoate (0.18 g, 0.62 mmol) in EtOH (10 mL) was flushed with N2. Pd/C (0.018 g) was added and the reaction was flushed with H2 for 2 hours. Then, the resulting mixture was filtered through celite and the filtrate was concentrated. Purification by flash chromatography, eluting with 15% to 60% of 92:7:1 CHCb/MeOH/concentrate NH4OH in CH2CI2, afforded methyl 2-amino-5-(methylsulfonamidomethyl)-benzoate (0.085 g, 53%).[0189] To a solution of methyl 2-amino-5-(methylsulfonamidomethyl)benzoate (0.085 g, 0.33 mmol) in THF (7 mL) and H2O (3 mL) was added LiOH-H2O (0.028 g, 0.65 mol). The reaction was stirred at room temperature for 2 hours and then neutralized with 1 N HCI. The resulting aqueous solution was extracted with EtOAc. The organics were washed with brine, dried (Na2SO4), filtered, and concentrated, to afford 2-amino-5-(methylsulfonamidomethyl)benzoic acid (0.066 g, 82%).[0190] A solution of 2-amino-5-(methylsulfonamidomethyl)benzoic acid (0.066 g, 0.27 mol) in THF (5 mL) was treated with EDCI (0.062 g, 0.32 mmol), HOBT (0.044 g, 0.32 mol) and NMM (0.035 mL, 0.32 mmol.) The reaction was stirred at room temperature for 1.5 hours. Then, NH4OH (0.03 mL, 0.35 mmol) in H2O (0.03 mL) was added. The mixture was stirred at room temperature for 5 hours and then concentrated. Purification by flash chromatography, eluting with 92:7:1 to 7:2.5:0.5 CHCI3/MeOH/concentrated NH4OH, afforded 2-amino-5- (methylsulfonamidomethyl)benzamide (0.035 g, 53%).[0191] A mixture of 2-amino-5-(methylsulfonamidomethyl)benzamide (0.035 g, 0.14 mmol), 4-hydroxy-3,5-dimethyl benzaldehyde (0.022 g, 0.14 mmol) and CuCI2 (0.039 g, 0.28 mmol) in EtOH (5 ml_) was refluxed for 3 h, then concentrated in vacuo. Purification by flash chromatography on silica gel, eluting with 92/7/1 CHCI3: MeOH xoncentrated NH4OH, followed by reverse-phase chromatography, eluting with 10% to 50% CH3CN in H2O with 0.1% TFA, and finally flash chromatography on silica gel, eluting with 7:2.5:0.5 CHCI3/MeOH/concentrated NH4OH, afforded the title compound (0.030 g, 57%) as a white solid. 1H NMR (300 MHz, DMSO-Cf6): delta 8.09 (s, 1 H), 7.83-7.90 (m, 2H), 7.65-7.78 (m, 3H), 6.81-7.54 (m, 2H), 4.30 (d, J = 6.2 Hz, 2H), 2.91 (s, 3H), 2.24 (s, 6H). ESI MS m/z 374 [M+H]+. |
| 40% |
With N-Bromosuccinimide; dibenzoyl peroxide; In chloroform;Reflux; |
To a solution of <strong>[20587-30-8]methyl-5-methyl-2-nitrobenzoate</strong> (2.3 g, 11.8 mmol) in CHCl3 (150 mL) was added NBS (5.3 g, 30.0 mmol) and benzoyl peroxide (0.285 g, 1.2 mmol). The reaction was heated at reflux temperature overnight. Then, the resulting mixture was washed sequentially with H2O, Na2CO3, and brine. The organic layer was then dried (Na2SO4), filtered, and concentrated in vacuo. Purification by flash chromatography on silica gel, eluting with 5% to 20% ethyl acetate/heptane, afforded methyl 5-(bromomethyl)-2-nitrobenzoate (1.3 g, 40%) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping